The genetic information of a mammal cell is stored in two types of molecules: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Understanding the susceptibility of these molecules to radiation damage helps to more accurately estimate dose rates for cancer treatment.
While most research has focused on the effects of radiation on the DNA that is stored in the cell nucleus, our research shows that there are also significant effects on genetic material other than the cell nucleus i.e. mitochondria, the energy-producing units of the cell. Our research shows that radiation has complex interactions with genetic material outside the nucleus and causes responses thatare not yet captured by current dose estimation methods.
Genetic material outside the cell nucleus
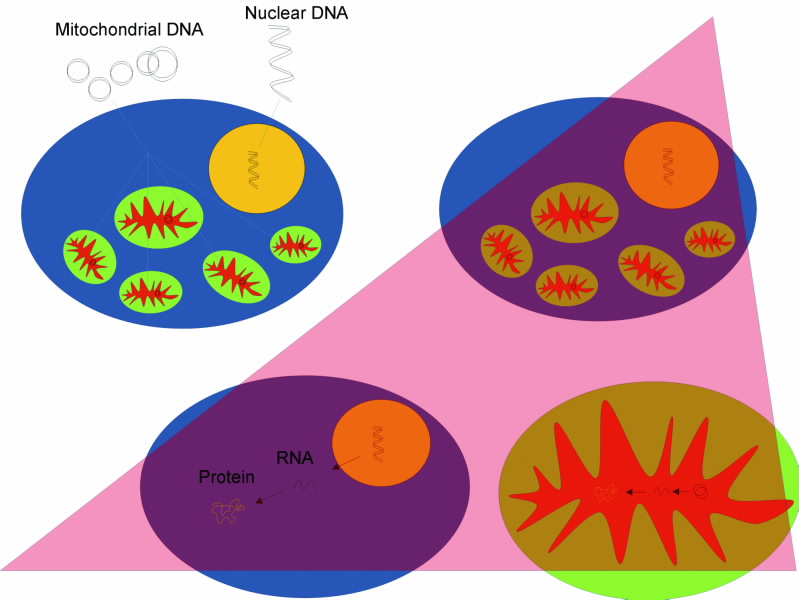
Research into the impact of radiation on living matter is usually focused on its effect on the genetic material inside the cell nucleus, i.e. chromosomal DNA, and its repair mechanisms. However, within the cytoplasm of the cell is an organelle, the mitochondrion which contains a small chromosome of its own, a small circular DNA coding for 37 genes.
Sometimes referred to as the “power plants of the cell”, mitochondria generate adenosine triphosphate (ATP) as a source of energy. In addition to supplying cellular energy, mitochondria are involved in a range of other processes. They are also important for cell signalling, differentiation, growth, cell death, and the cell cycle. Figs. 1A and 1B show electron microscope images of a cell (macrophages) containing numerous mitochondria; Figs.
1C to 1E show mitochondria in different functional states after cell activation. Fig. 2 shows mitochondria stained by a fluorescent dye. In many cell types, mitochondria take up a greater portion of the cell volume than the nucleus, as can be seen in Figs. 1 and 2. Thus, the likelihood of radiation being received by mitochondria is expected to be greater than for the nucleus.
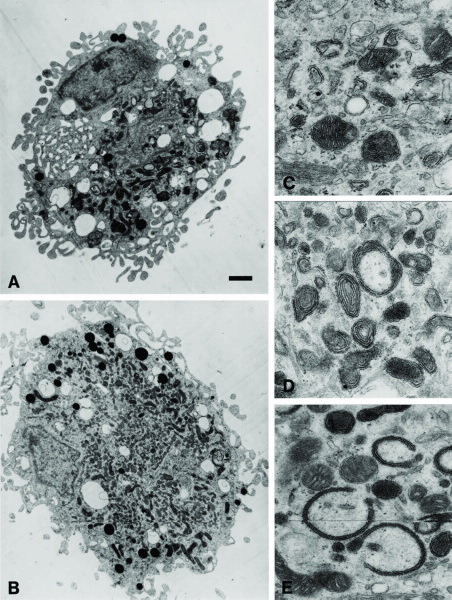 |
| A mammalian brain macrophage (A&B): The arrow point to the cell nucleus. The mitochondria are the dark/elongated structures inside the cell. Scale bar: 1um. The shape of the mitochondria reflects the functional state of the cell; (c) mitochondria in a resting cell; (D&E) mitochondria in activated cells. |
There are two types of genetic material:
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA serves as the genetic blueprint. DNA is transcribed into RNA which is the messenger that translates into proteins. DNA is a relatively robust double-stranded, structure, which is either helical in the nucleus or circular in mitochondria. In contrast, RNA is single-stranded making it more vulnerable to various stresses, including radiation, see Fig. 3.
Despite the importance of RNA (for protein synthesis) and mitochondria (for energy production and hence cell survival), radiation-induced changes to the genetic material in mitochondria has so far received little attention.
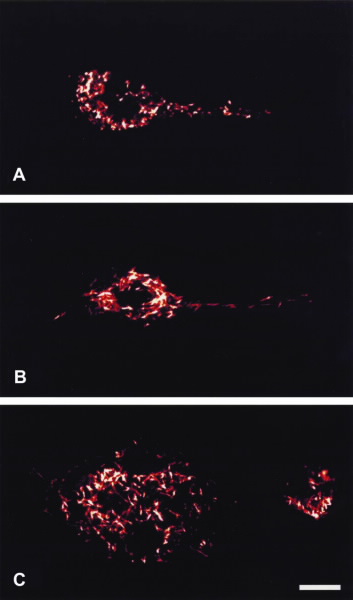 |
| Visualization of mitochondria stained with a fluroescent mitochondrial dye. In contrast to a resting cell (a), activated cells have mitochondria, that form a dense network of often large elongated mitochondria (B&C). Scale bar: um. |
Experimental gamma irradiation of cells
Mammalian cells (of lymphocytic lineage) were irradiated at room temperature using a cobalt-60 irradiator. 60Co emits gamma rays, ionising, high-frequency electromagnetic radiation (similar to X-rays). Our control samples were kept at room temperature and were not irradiated. RNA and DNA extractions from the irradiated and non-irradiated cells were performed immediately after irradiation. The extracted RNA and DNA were analysed by quantifying the amount of mitochondrial gene present after irradiation using quantitative real-time polymerase chain reaction. This technique uses repeated amplification cycles to amplify minute quantities of target gene from a pool of nucleic acids (RNA or DNA template).
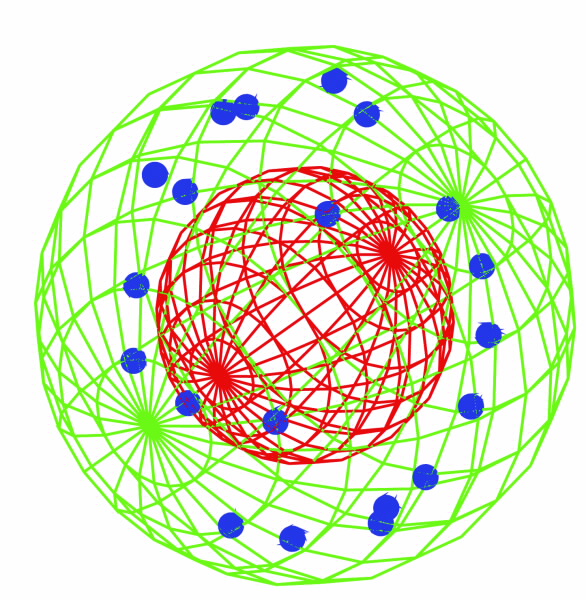 |  |
(a) A schematic view of the cell and the trges of our radiation experiment. Nucleus (red sphere) and mitochondria (blue spheres) are randomly distributed within the cell. (b) Irradiation effects all components within the cell i.e DNA in both nucleus and mitochondria. Note that in mitochondria, the transcription from DNA to RNA and then translation into protein occurs within the space othe organelle. |
So far we have analysed 18 mitochondrial genes. Fig. 4 shows the results: After an irradiation dose of 100 Gy, 12 out of the 18 mitochondrial genes amplified from the mitochondrial RNA template showed an increase in the amplifiable amount. This suggests that, in response to this particular radiation dose, there was already an early increase in the rate of transcription from the mitochondrial DNA into mitochondrial RNA during the period of irradiation itself (a period of ~2.5 minutes in our experiments).
Interestingly, after irradiation, mitochondrial DNA also responded with an increase in the detectable amount. Unlike mitochondrial RNA, the multiplication of mitochondrial DNA was detectable only at the higher dose of 1000 Gy. We are currently dissecting the specific mechanism, such as time courses, temperature dependency and others, that lead to the changes in small mitochondrial chromosomes. This analysis also extends to the question of which pathways are likely to be affected by the differential regulation of mitochondrial RNA expression after radiation exposure.
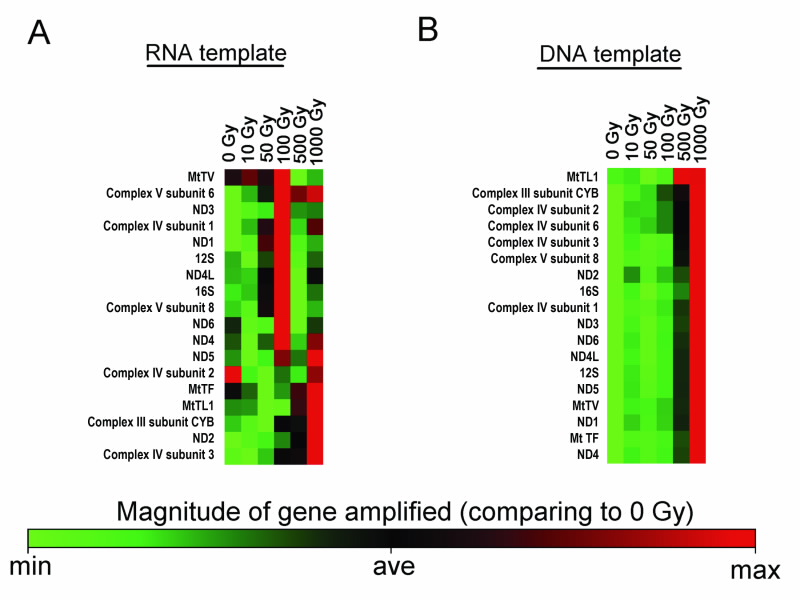 |
| The acute response of mitochondrial RNA and DNA to gamma radiation. Genes were amplified from (a) RNA (b) DNA template using a quantitative real-time polymerase chain reaction. The heat maps graphically represent the relative amount of the 18 selected mitocondrial genes amplified from each type of the template at each tested dose, when compared to the non-irradiated sample. The genes are sorted according to their overall simiarity in quantity. |
In summary, our data show that cell irradiation leads to a rich set of Complex responses of extra-nuclear DNA and RNA after gamma radiation responses that relate to genetic material outside the cell nucleus. Since extra-nuclear DNA and RNA are crucial for vital cell functions, future radiation dose models will need to take into account the amount of radiation received by mitochondria as well as their complex dosedependent response to it, see Fig. 5.
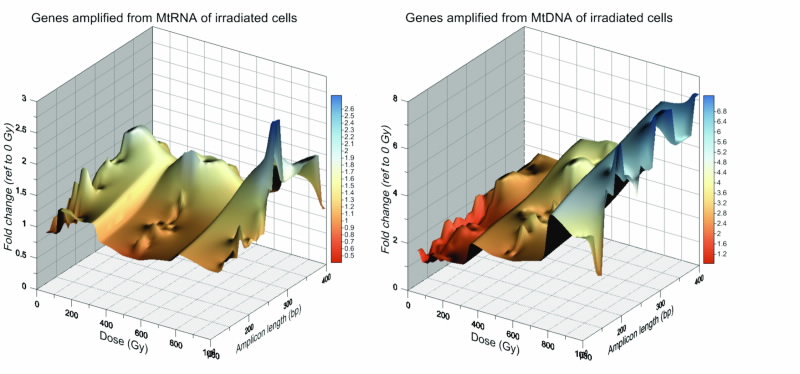 |
| The relationship between the level of gene detectable (fold change reference to 0 Gy control), dose- delivered and the length of the amplified gene (amplicon length). 3D plots on the profiles of dose-dependent changes in amplifiable gene from (a) mitochondrial RNA and (b) mitochondrial DNA, demonstrating the complex highly differented response that varies significantly between different genes. |
Authors
Winnie W.Y. Kam1, Aimee L. McNamara2, Connie Banos1, Sohil Sheth1, Justin B. Davies1,2, Richard B. Banati1,2
1ANSTO, 2University of Sydney.
References
- Banati, R.B., Egensperger, R., Maassen, A., Hager, G., Kreutzberg, G.W. and Graeber, M.B., Mitochondria in activated microglia in vitro, Journal of Neurocytology, 33(5), 535-541 (2004).
Published: 17/02/2012


