Ionic liquids are simply salts with a melting point below 100ºC. Many melt below room temperature, hence their name. Their rather varied physical and chemical properties are interesting because they can be employed in improving various chemical processes, such as the electrodeposition of metals.
Ionic liquids have attracted particular interest because they allow aluminium to be electrodeposited at room temperature. We have used modelling combined with neutron and X-ray reflectometry to examine the structure of ionic liquids at the air-liquid and electrode-liquid interfaces to understand the electrochemical processes.
Ionic liquids
Most ionic salts have very high melting points as a consequence of strong ionic bonding between the small cation and anion (for example NaCl has a melting point of 801ºC). However, if the ionic bonds are weaker it is possible for the ionic salt to be molten at much lower temperatures (<100º Celsius), such salts are referred to as ionic liquids. They are typically composed of a large nasymmetric organic cation and a smaller organic or inorganic anion.
Ionic liquids have many important physical and chemical properties that are extremely attractive to industries. These properties include low vapour pressure, high viscosity, high temperature and nelectrochemical stability and a wide electrochemical nwindow. It is this last property that has spurred a ncollaborative research project between ANSTO and CSIRO Minerals over the past four years.
The reason is that current production of aluminium requires a huge amount of energy because alumina has to be dissolved in molten cryolite at ~960º Celsius before electrodeposition can occur.
This expensive process is required because aluminium cannot be electrodeposited from water because its reduction potential is outside the electrochemical window of water.
However, electrodeposition should be possible from some ionic liquids as their electrochemical windows encompass the reduction potential of aluminium, giving the tantalizing vision of large scale aluminium production at room temperature (no massive heating bills).
One candidate for this process is 1-butyl-1- methylpyrrolidinium bis(trifluoromethylsulfonyl)imide n(P14Tf2N). In our project, we performed a fundamental nexamination of the interfacial structure of this liquid at the air-liquid and electrode-liquid surfaces.
Indeed, the behaviour of ions at charged interfaces is already well known for aqueous electrolytes, with theory from Helmholtz and Gouy and Chapman (in the early 20th century), but much less so for ionic liquids. The behaviour of this structure plays an essential role in all interfacial electrochemical processes.
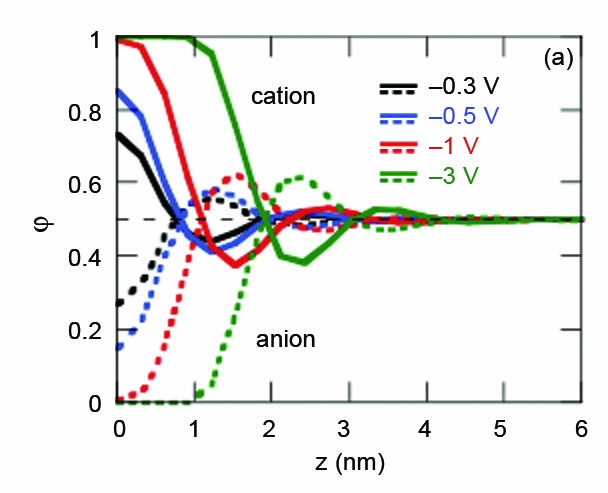 |
| Fig 1. Profile of the volume fraction of cation and anion at the interface with different surface potential values. Taken from [1]. |
Modelling approaches
The first approach used theoretical modelling techniques to predict the equilibrium structure of ionic liquids at a charged electrode surface (also termed the electrical double-layer) and the correlation between the electrical double-layer and its electrochemical capacitance [1].
The capacitance of the electrical double-layer is an indication of how the electrostatic potential of the electrode surface is screened by the ions that accumulate there. The modelling was able to predict nthe volume fraction profiles of the cation and anion, nrevealing an oscillatory nature that depends on the nsurface potential of the electrode surface, Fig. 1.
Quite simply, as electrode potential increases there is a greater excess of counter ion in the layer adjacent to the electrode and the ordering increases.
Subsequent modelling investigated the effect of size asymmetry on the electrical double-layer structure, revealing that the ordering becomes damped when size nasymmetry between the cations and anions is larger [2].
Experimental approaches
To complement the information obtained from the theoretical approaches we used neutron and X-ray reflectometry at ANSTO.
These techniques are ideal for studying chemical composition gradients across interfaces, especially when the interface of interest is buried – such as the electrode-ionic liquid interface. The initial X-ray reflectometry work found [3] that cations preferentially adsorb at the air-liquid boundary.
When water impurities were doped into the ionic liquid they also (ionic liquids are very hygroscopic) adsorbed at the air-liquid boundary, but had a non-monotonic effect on the overall thickness of the surface layer.
The trend in surface thickness was able to explain the trend in surface tension of ionic liquid-water nmixtures reported in the literature. Understanding where water impurities concentrate is important as they greatly affect the electrochemical behaviour of ionic liquids at solid electrodes.
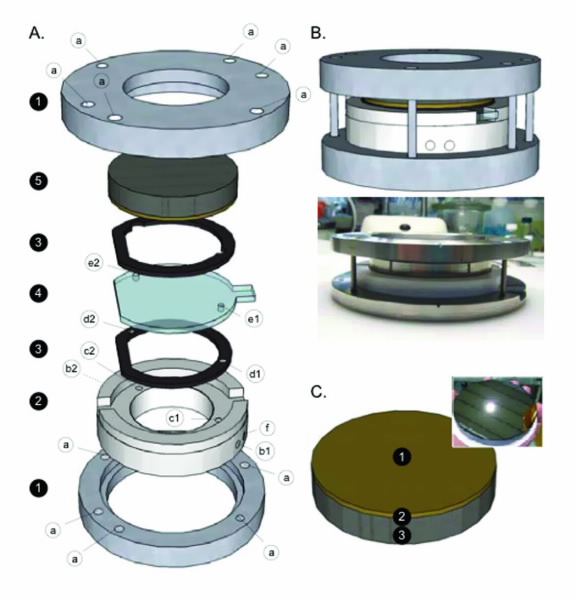
|
| Fig 2. The in situ electrochemical cell used for the investigation of the electrical double-layer structure at different electrode potentials. The silicon wafer is 100 mm in diameter. |
In order to study the ionic liquids at the electrodeliquid interface we performed several neutron reflectometry experiments on Platypus, ANSTO’s neutron reflectometer. By constructing a unique electrochemical cell [4], Fig. 2, we were able to ncharacterise the electrical double-layer at different surface potentials.
This data is still in the process of being analysed, nbut early results give the unusual conclusion that cations preferentially adsorb even at positive surface potentials.
Authors
Andrew Nelson1, Yansen Lauw1,2, Mike Horne2, Theo Rodopoulos2
1ANSTO, 2CSIRO Process Science and Engineering, Clayton South, Australia
References
- Lauw, Y., Horne, M. D., Rodopoulos, T., & Leermakers, F. A. M. (2009). Roomtemperature ionic liquids: excluded volume and ion polarizability effects in the electrical double-layer structure and capacitance. Physical Review Letters, 103(11), 117801-117804.
- Lauw, Y., Horne, M. D., Rodopoulos, T., Nelson, A., & Leermakers, F. A. M. (2010). Electrical double-layer capacitance in room temperature ionic liquids: ionsize and specific adsorption effects. Journal of Physical Chemistry B, 114(34), 11149-11154).
- Lauw, Y., Horne, M. D., Rodopoulos, T., Webster, N. A. S., Minofar, B., & Nelson, A. (2009). X-Ray reflectometry studies on the effect of water on the surface structure of [C4mpyr][NTf2] ionic liquid. Physical Chemistry Chemical Physics, 11(48), 11507-11514.
- Lauw, Y., Rodopoulos, T., Gross, M., Nelson, A., Gardner, R., & Horne, M. D. (2010). Electrochemical cell for neutron reflectometry studies of the structure of ionic liquids at electrified interface. Review of Scientific Instruments, 81(7), 074101.
Published: 17/03/2011