 |
| ANSTO Biologist, Nick Howell's research is contributing to better treatments for diseases. |
As a result of many successful health strategies, technologies and initiatives, life expectancy in Australia is higher now than ever before. Although this is a good result, an increasingly ageing population comes with a number of challenges including a growing number of people with age related disabilities. Dementia is the most common cause of disability in Australians over the age of 65.
The term ‘dementia’ actually encompasses more than 100 degenerative disorders, including Alzheimer’s, Parkinson’s and Huntington’s diseases, that affect the central nervous system, and in particular the brain. In order to develop better treatments, we first need to better understand the basic underlying disease processes.
We know that calcium plays an important role in neurons, which are cells that are essential for a healthy functioning human nervous system. An alteration in calcium homeostasis is one of the events known to initiate irreversible deterioration of the nervous system, which ultimately leads to degeneration of brain tissue.
By measuring particle induced X-ray emissions in sections of inflamed rat brain tissue, ANSTO’s Nick Howell and his colleagues have been able to capture images of changes in calcium distribution. These images give potential insight into the early stages of dementia and help scientists better understand how these diseases start and progress. It is hoped that the results of this research will enable scientists to not only detect these diseases before symptoms are visible, but also contribute to the development of better treatments.
Neurodegenerative disorders
As in many other developed countries, increasing life expectancy through successful health strategies is giving Australia a significant and growing aging population. The impact of the aging population on the health system is significant, with dementia in particular being the greatest cause of disability in Australians over 65 years, and the fourth largest cause of disability overall The societal burden of dementia currently costs the health and aged care sector $4.9 billion per annum.
Neurodegenerative disease encompasses a spectrum of over 100 disorders, of which Alzheimer’s disease, Parkinson’s disease and Huntington’s disease are the most common. Younger and older populations are also impacted by neurological disorders such as stroke and epilepsy, with approximately 400,000 people impacted by each disease.
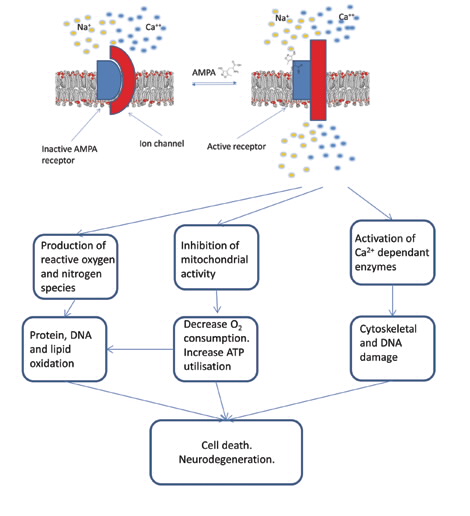
In order to develop treatments, we need to increase our understanding of underlying disease processes that are common to these conditions. Excitotoxicity is the pathological process by which nerve cells in the brain are damaged or killed by a disease process initiated by an excessive release of a compound in the brain that is used to communicate between specific neurons, called glutamate [1].
The resulting excessive stimulation of the receptor target for glutamate, allows the influx of calcium ions (Ca+2) through the outer membrane, which in turn stimulates a number of catabolic processes and results in an increase in the levels of free radicals and hence oxidative damage to proteins lipids and DNA. This results in an immune response within the affected area of brain, and eventually neurodegeneration [2].
 |
| Figure 1: AMPA- an excitatory amino acid analogue for the endogenous neurotransmitter glutamate. |
Ca+2: Friend or Foe?
Ca+2 ions are fundamentally important to cell survival. Intracellular calcium plays a great diversity of roles such as signal transduction, muscle contraction, neurotransmitter release and is a cofactor required for many enzymatic reactions [3]. Extracellular Ca2+ is an important cation for maintenance of electrical potential across cellular membranes, the driving force for many cellular functions.
Additionally Ca is fundamentally important in neurotransmission the process of chemical communication between neurons, which occurs at the synapse. Electrical communication from one neuron (presynaptic) to the next (postsynaptic) requires the release of a chemical (neurotransmitter) from the presynaptic neuron to bind to and activate the receptors of the adjacent neuron (postsynaptic).
This cascading event results in the propagation of signal and the creation of nerve pulses. The energy required for neurotransmission is stored as an electronic membrane potential across the external membrane of the neuron [2]. When the electrical signal arrives, influx of Ca+2 through voltage or ligand gated ion channels allows the efflux of neurotransmitters.
In excitotoxic brain injury, aspects of this process go wrong. Large fluxes of concentrations of Ca+2 within cells are a key feature of the initiation of the excitotoxic process. Excitotoxicity occurs when receptors for the neurotransmitter glutamate are overstimulated, leading to an excessive influx of extra cellular Ca2+.
This increase in intracellular Ca2+ leads to the over-activation of a number of enzyme families of proteases, nucleases and lipases which then alter or damage vital components of the cell including membranes, proteins and DNA [4]. Increased intracellular Ca2+also removes the membrane potential across the inner mitochondrial membrane causing the mitochondria to swell and release various reactive oxygen species; see scheme in Fig. 1 [5].
The reaction of the immune response within the brain can be visualised via microglia, brain macrophage cells, which migrate to the site of injury and engulf the dead and dying neurons [5].
Inducing excitotoxic injury
We have employed a well-characterised model of excitotoxicity in these studies, in which the stimulus, the excitatory amino acid, α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid (AMPA), (see Fig. 2) was injected stereotaxically into the striatal brain region (responsible for cognitive processes) of male Wistar rats (but only on one side) under anaesthesia.
After recovery, the excitotoxic process and resulting neuroinflammation proceeds for seven days after which the samples are collected [6].
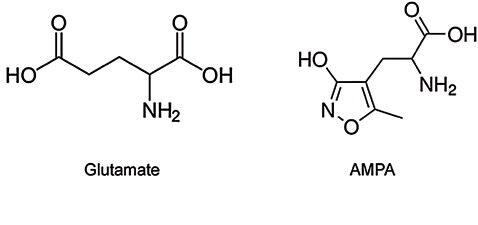 |
| Figure 2: AMPA- an excitatory amino acid analogue for the endogenous neurotransmitter glutamate. |
Imaging: altered calcium homeostasis using μPIXE
The ANSTO high-energy heavy-ion microprobe and particle induced X-ray emission device (PIXE) offers a unique capability of generating quantitative ‘maps’ of the distribution of elements in post-mortem tissue. Using other histopathological methods, such as immunohistochemistry which shows neuroimmune activation, we can correlate the images with other processes coincide with the time the sample is taken.
We used micro-PIXIE (μPIXE) to image the distribution of calcium concentration in cryo-sectioned brain tissue. Using a 3 MeV proton beam with a spot size of about 7–10 μm and a beam current of 0.5–0.8 nA, various atoms within the tissue section are excited and the resulting X-ray fluorescence spectra recorded.
As shown in Fig. 3, the characteristic X-ray lines in the spectra clearly show fluorescence of P, S, Cl, K and Ca, which are critical electrolytes in all cells. We also observed characteristic lines for Fe, Zn, Mn and Br, some of which are present as trace elements. By fitting the known spectral profiles for these elements, quantitative maps of the distributions of them within the brain section can be reconstructed, as shown in Fig. 4) [7].
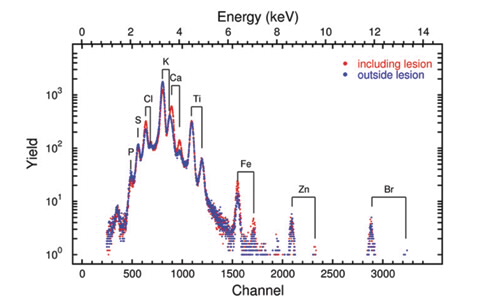 |
| Figure 3: μPIXE spectra taken over a 2 x 2mm2 area in the region of the neuroinflammation and for comparison a spectrum over the same area on the right brain hemisphere. |
The reconstructed image illustrates significant increases in calcium concentration within the right striatum, where excitotoxicity was elicited.
Reductions in K and S are seen within the site of excitotoxic injury, indicating reduced cellular density as a result of neurodegeneration. Adjacent brain sections displayed significant up-regulation, demonstrated with immunohistochemistry, of integrin αM (Fig. 5), a cell surface protein involved in the adhesion and migration of immune cells. This indicates that the endogenous immune cells of the brain, the microglia, are ‘activated’ in response to neurodegeneration within the area.
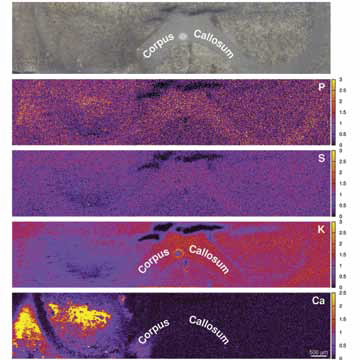 |
| Figure 4: Elemental maps of Ca, K, S and P, together with an optical image of the analysed area. |
What can we learn from calcium?
Imaging elemental distributions in the brain using μPIXE has demonstrated large fluxes of calcium concentration, in response to excitotoxic injury.
Currently, excitotoxic injury is visualised by analysing the level of neuroinflammation. As calcium influx is the major initiating factor of excitotoxic injury, this method—developed at ANSTO—has the potential to visualise the excitotoxic process before the resulting neuroinflammation.
This provides another unique approach for assessing disease, in particular the correlation of in-vivo pathology with post-mortem alterations. Invitro methods on biopsy material are the mainstay of histopathological assessment of disease clinically. Validating the use of in-vivo imaging, such as positron emission tomography(PET), from biopsy histopathology data allows greater power in assessing disease progression in patients compared to invasive procedures or situations where biopsies are not possible.
It is hoped that imaging calcium elemental distribution will give the ability to visualise the early time points and origin of the injury. This approach can be used as an indicator of ‘prodromal’ or disease initiation before symptoms appear in diseases (initially in animal models) such as:
- Neurodegenerative disease
- Epilepsy
- Amphetamine related cognitive dysfunction
This will allow a greater understanding of the initiating factors and brain regions in response to various stimuli thereby increasing our understanding of the role of this fundamental process in many related pathologies.
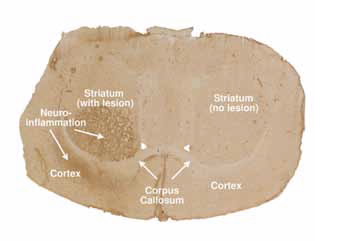 |
| Figure 5: Optical image of a rat brain section. The image clearly shows the region of the neuroinflammation in the AMPA lesioned rat. The sample was stained by OX-42 immunohistochemistry, a biomarker for microglial activation resulting from localised excitotoxic damage of neurons. |
Authors
Nicholas Howell1, Paul Callaghan1, Rainer Siegele1, Richard Banati1,2
Nicholas Howell1, Paul Callaghan1, Rainer Siegele1, Richard Banati1,2
1 ANSTO, 2 University of Sydney, New South Wales
References
- Bondy, S.C. and C.P. LeBel, The relationship between excitotoxicity and oxidative stress in the central nervous system. Free Radical Biology and Medicine, 1993. 14(6): p. 633-642.
- Patel, D.R. and C. Feucht, Basic Concepts of Neurotransmission. Pediatric Clinics of North America, 2011. 58(1): p. 21-31.
- Williams, R.J.P., The evolution of the biochemistry of calcium, in New Comprehensive Biochemistry, K. Joachim and M. Marek, Editors. 2007, Elsevier. p. 23-48.
- Gümrü, S. and F. Arýcýoðlu, Ampakines: Selective AMPA receptor modulators with potential benefits. MÜSBED, 2012. 2(4): p. 143-148.
- Nam, M.-K., et al., Essential roles of mitochondrial depolarization in neuron loss through microglial activation and attraction toward neurons. Brain Research, (0).
- P.D. Callaghan, et al. in World Molecular Imaging Congress. 2009. Montreal, Canada
- Hackett, M.J., et al., Investigation of the mouse cerebellum using STIM and μ-PIXE spectrometric and FTIR spectroscopic mapping and imaging. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2011. 269(20): p. 2260-2263.
Published: 14/03/2014


