Treating cancer patients with drugs requires a fine balance between killing tumour cells without damaging normal cells, and managing the body’s clearance of anti-cancer drugs so as to be effective without being toxic to the patient.
 |
If the body clears chemotherapy drugs too quickly, the tumour attacking action is lost, while slower metabolism of anti-cancer drugs results in higher concentrations of these drugs in the body which in turn causes toxicity and forces treatment to be delayed or stopped. A better understanding of how to achieve this fine balance will help in the treatment of cancer patients.
Arvind Parmar1, Frederic Boisson1, Marie-Claude Gregoire1, Andrew Katsifis1, John Allen2, Graham R. Robertson2, Stephen Clarke3 1ANSTO, 2ANZAC Research Institute, University of Sydney, New South Wales 3University of Sydney, Royal North Shore Hospital, New South Wales. |
The challenges of chemotherapy
Anti-tumour action is lost if the drug is cleared too rapidly, while slower metabolism of chemotherapy drugs results in higher concentrations in the body which, in turn, causes toxicity resulting in the delay or termination of treatment. One factor known to contribute to altered drug clearance in cancer patients is inflammation associated with the growth of tumours.
We have previously found that the presence of a tumour causes a reduction in the enzymes and drug transporters in the liver that take up drugs from the bloodstream into organs such as liver, brain and bone marrow as well as pumping toxins out of the body. This reduction results in slower clearance of drugs. Because of this some patients develop adverse side effects from chemotherapy [1].
| Imaging scanner | Protocol | Animal model | Radiotracer |
Inveon Trimodality PET/SPECT/CT | SPECT: 99mTc-60 projection, ROR 30mm, 1 revolution, 15s projection, 20mm travel, 9 x 20mins scan (3hr total scan time) CT: 220° rotation 220 Projection, 4x4 bin, 113.22μm effective pixelsize, 600ms exposure 50kVp, 500μA, 3072x2048, DS1. | Balb/c xDBA mice(n=3) engrafted with Colon 26 (C26) tumour cells Balb/c xDBA healthy normal mice (n=3) as a control group | 30 Mbq/100μl of 99mTcsestamibi intravenously, which is exported from cells via drug transporters |
How can molecular imaging help?
The imaging system is versatile as it can combine three techniques Single-Photon Emission Computed Tomography (SPECT), Positron Emission Tomography (PET) and Computed Tomography (CT) in a single integrated scanner (see Table 1 for details). This provides three-dimensional imaging of multiple dynamic volumes and that are required for our measurements of bio-distributions.
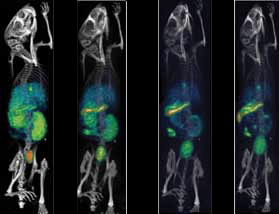 | 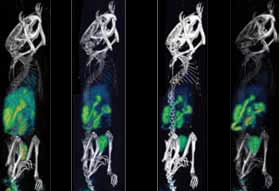 |
| Figure 1: The SPECT/CT images of 99mTc sestamibi confirm predominant uptake in liver, kidneys and bladder at initial time points. At later time points, remarkable activity can be seen in intestine and urinary bladder. The increased retention of sestamibi in various organs in tumoured mice compared to normal mice is apparent over 3hrs. | |
Imaging the biodistribution
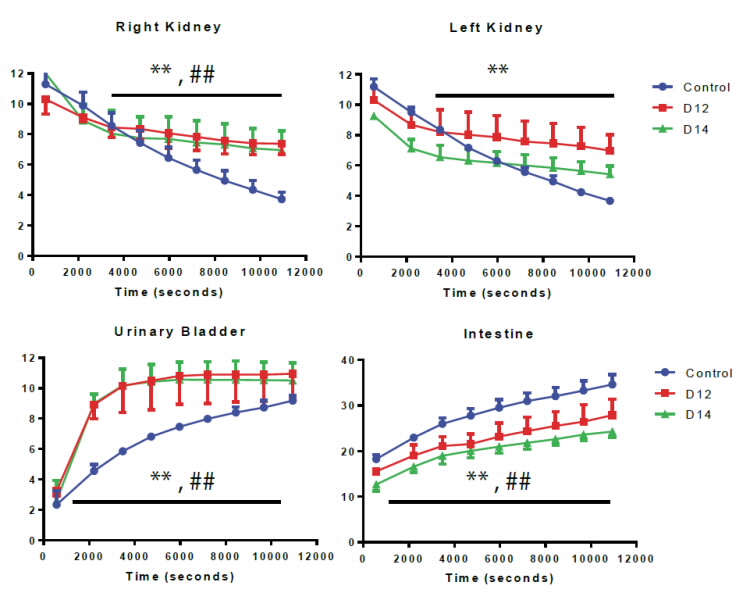 |
Figure 2: 0-3 hrs time activity curve of Tc-99m sestamibi for various organs in normal mice, day 12 (D12 in red) and day 14 (D14 in green) tumour mice. (n=3). Data expressed as mean counts in tissue + Standard Error from Mean).. ** p < 0.05 D12 group compared to control, ## p < 0.05 D14 group compared to control. |
The Future
We are in the initial stages of understanding how to monitor the clearance of chemotherapeutic drugs based on these results. The differences in drug biodistribution and pharmacokinetics for tumour-bearing versus control mice are significant and lay the foundations for subsequent experiments aimed at reversing inflammatory signalling associated with cancer in mice.
We now plan to perform imaging with first line anti-cancer drugs as a prelude to clinical studies in human cancer patients to identify those at risk of excessive toxicity. We aim to characterise the functional impact of tumour-induced inflammation on anti-cancer drug pharmacokinetics and identify strategies to normalise drug handling, leading to improved individualisation of anti-cancer drug dosing, and hence safer and more effective chemotherapy for patients.
Progressively, we hope to establish diagnostic biomarkers to identify patients at greater risk of toxicity from chemotherapy prior to its use, enabling better surveillance and more appropriate use of prophylactic antibiotics and growth factors. The new knowledge generated from these studies has the potential to fundamentally change cancer chemotherapy dosing strategies to improve the safety and efficacy of cancer treatment.
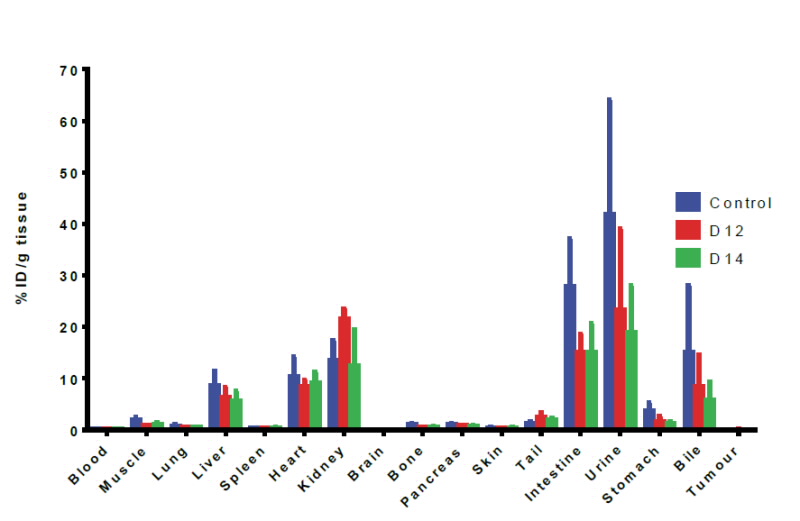 |
Figure 3: Biodistribution data of Tc-99m sestamibi at the 3hour time point for various organs in normal mice, day 12 (D12 in red), and day14, (D14 in green), Tumour mice. (n=3), data expressed as Mean ID/g+ SEM (Mean injected dose/gram of tissue+ Standard Error from Mean). |
Acknowledgement
This study is a component of NHMRC project grant ID#632848 –”Improving the use of chemotherapy” 2010-2012.
References
- Sharma R, Kacevska M, London R, Clarke SJ, Liddle C, Robertson G. Down regulation of drug transport and metabolism in mice bearing extrahepatic malignancies. British Journal of Cancer. (2008);98:91–7.
- Boisson F, Zahra D, Parmar A, Gregoire M.C, Meikle S, Hamse H, Reilhac A. Imaging capabilities of the Inveon SPECT system using single and multi-pinhole collimators. Journal of Nuclear Medicine (Accepted)


