New innovations in waste reduction and recycling that are being developed at ANSTO have the potential to minimise reprocessing requirements of future nuclear fuels.
Selective separation of aqueous elements is a vitally important and industrially significant process not only with regards to reducing the volumes of nuclear-waste materials, but also for extracting industrially important elements: all rely upon the ability to remove a specific element from a complex mixture of elements.
For this purpose, we create new functionally designed materials for selective separation, in particular, within the context of the nuclear industry.
Selective separation in relation to the nuclear industry
Nuclear energy increasingly represents an important option for generating clean CO2-free electricity generating increased interest in innovative nuclear reactors such as “Gen (IV)” reactors [1].
The concept of these reactors implies recycling of irradiated fuel, waste-reduction strategies and improved waste technologies – also called closed fuel-cycles.
Currently, reprocessing of used nuclear-fuel is carried out in several countries including France, UK, Russia and Japan using the PUREX (Plutonium and Uranium Extraction) process.
However, recovery of the other transuranic elements, i.e. Neptunium (Np), Americium (Am) and Curium (Cm), from used fuel has not yet been achieved industrially.
As Np, Am and Cm contribute significantly to the lifetime and radiotoxicity of nuclear waste they are targets for removal.
It may also be possible to recover and re-use some of the other fission products present in used nuclear-fuel, for example 137Cs and 90Sr are industrially relevant products (e.g. for use in gauges, as radioactive tracers and 90Sr is used in medicine).
Thus, separation of 137Cs and 90Sr from used nuclear-fuel is under development - a topical area of research. Lanthanides (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er) are another industrially relevant product: used in magnets, hybrid cars, LEDs for TV and computer screens, and similar.
As the demand and cost for these elements has increased dramatically (up to 750%) over the last year their extraction is a potential new resource.
Elements can be separated by precipitation, fractional crystallisation, solvent-solvent extraction, liquid-solid extraction, electrochemical methods and others.
Current commercial nuclear-separation technology is based upon solvent-solvent extraction, but this technology is inefficient and suffers complications due to radiation-induced decomposition.
Consequently, new separation methodologies are searched for. We are currently investigating two such methodologies: liquid-solid extraction and electrochemical methods. The latter involves investigation into the potential of pyroelectrochemical processes based on molten alkali salts; this is studied in partnership with the ACSEPT (Actinide reCycling by SEParation and Transmutation) programme of the European Union.
However, in the following we report on liquid-solid extraction where we develop new sorbent materials that can withstand the imposed radiation and improve separation efficiencies.
The separation-science strategies
Liquid-solid extraction via column chromatographic methods (solid phase is packed into a column and liquid phase is pumped through) offers a simple, safe and economical alternative to current solvent-solvent separation technologies (see Fig. 1 in the image library below).
However, the particular environments encountered in nuclear systems (temperature, acidity) and radioanalysis means that any promising materials need to pass rigorous testing for all these requirements. To address this complex mix of requirements a multifunctional material is required.
This demands an interdisciplinary approach to materials selection and evaluation. For example, the solid-sorbent material in a chromatographic column needs to have high selectivity and allow a non-restricted flow of the liquid through the column as well as chemical and radiolytic stability.
Once sorption has occurred the solid-matrix material can be either; (i) converted to a waste form suitable for safe disposal, or (ii) used to elute a pure form of the extracted element, particularly if the separated element is industrially useful.
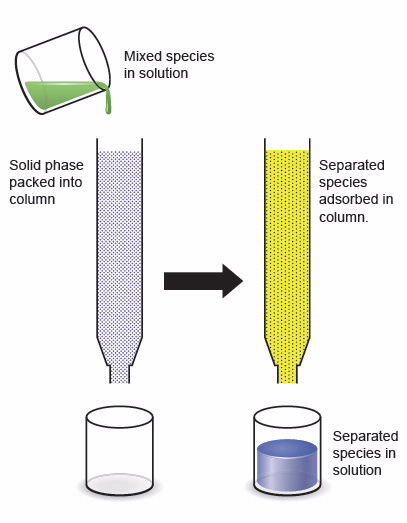 |
| Fig 1. Illustration of column chromatographic separation process; a mixed solution is added to the top of a column of solid phase, as the solution passes through the column the element of interest is retained within the solid phase and all other components are eluted from the bottom of the column giving a means of separating components in a solution. |
From macro- to meso-porosity
Producing the solid matrix in the form of beads imparts the desired characteristics for large-scale ion-exchange applications with ease of use and ease of flow. We have manufactured mixed titanium-zirconium oxide beads with the desired wide range of porosities from macro-to-meso, illustrated in Fig. 2.
But why mixed titanium-zirconium oxide compositions?
They allow potential inclusion in waste-form materials or can be used as transmutation matrices. The radial macroporous bead structure was imparted using polyacrylonitrile templating techniques and mesoporosity was generated using a secondary templating methodology.
The desired mesopore architecture can be built through evaporation-induced self-assembly technique (EISA) using a variety of supramolecular templates [2,3].
With this technique we tailor the binding sites through both channel size and surface charge and allowing the material and its properties to be tuned to a particular application.
Essentially, this results in a hierarchy of porosity from macro-to-meso materials with enhanced mass-transfer kinetics relative to materials that only had esoporosity of one size regime [4]. It should be pointed out that rapid adsorption kinetics is highly desirable in nuclear and other separation applications.
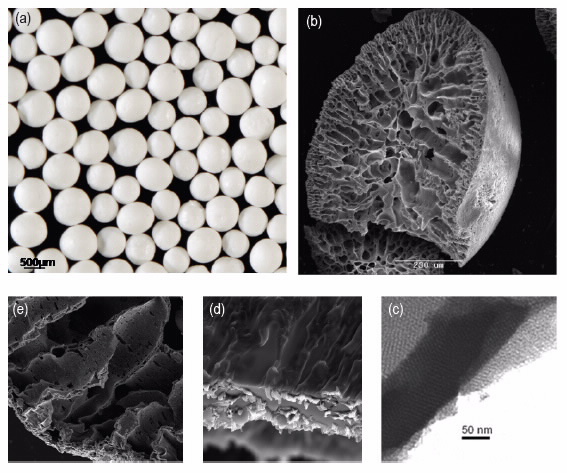 |
| Fig 2. (Clockwise starting top left): Bead structures of selective sorbent materials: (a) optical micrograph of the beads, (b) Scanning electron microscope (SEM) of a cross section of a bead showing the radial porous nature, (c) SEM-increased magnification, (d) SEM, (e) transmission electron microscope (TEM) of the internal surface of the beads showing the ordered mesopores. |
The key to selectivity
Mesoporous oxide materials can impart selectivity through size and charge, but greater chemical selectivity can be generated by attaching specific organic ligands to the surface, i.e. a surface offers a specificity (or preference) for the desired species to be attached.
For example, the use of amino trismethylenephosphonic acid [5] on the mixed oxide beads proved promising for lanthanide adsorption.
We call this ‘functionalising’ material and in this particular case surface functionalisation. We are now working on establishing selectivity in the presence of competing cations.
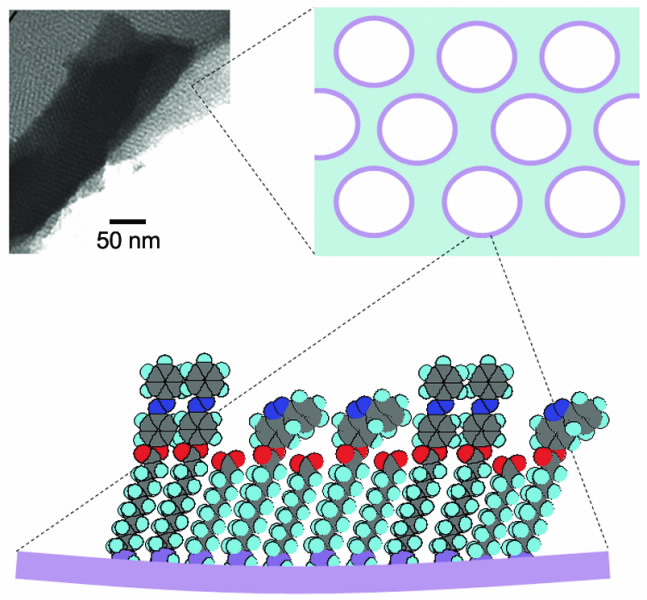
An organic ligand for functionalisation consists of three distinct parts: the surface binding group to attach to the mesopore, a linker, and the functional ligand itself (see Fig. 3).
Consequently, our research is concentrated on three main areas:
- Surface binding, how we chemically react with the mesopore surface;
- Self assembly processes for enabling the packing of the functional molecules inside the pores; and
- Synthesis of selective ligands that, once bound to the surface, remain available for co-ordination to the metal species of interest.
We pursue organic synthesis of molecules that consist of all three parts. In addition, we continue to investigate the synthesis and structure of mesoporous oxides and initiate investigations into alternative mesoporous-structures based upon carbon, titanium carbides and zirconium carbides.
These chemical compositions are important to new waste-form structures currently under development, which have the potential to minimise reprocessing requirements of future nuclear fuels.
|
| Fig 3. Illustration of surface functionalisation and its hierarchy. The internal surfaces of the mesopores are functionalised via self-assembly of organic chains which bind to reactive groups on the surface via a binding group. Selective ligands are then facing the interior of the mesopore available for binding of metal ions of interest. |
Authors
Tracey Hanley1, Jessica Veliscek-Carolan1, Nick Scales1, Zaynab Aly1, Ces Fabian1 and Victor Luca2
1ANSTO, 2Cómision Nacional de Energia Atómica, Argentina
References
- Olander, D. (2009). Nuclear fuels – present and future. Journal of Nuclear Materials, 389(1), 1-22.
- Luca, V., Drabarek, E., Griffith, C. S., & Hanley, T. L. (2010). Understanding the supramolecular self-assembly of zirconium titanate mesophases formed from the poly(ethylene oxide) surfactant brij-58. Chemistry of Materials, 22(13), 3834-3842.
- Luca, V., Soler-Illia, G. J. A. A., Angelomé, P. C., Steinberg, P. Y., Drabarek, E., & Hanley, T. L. Striving for order and compositional homogeneity in bulk mesoporous zirconium titanium mixed metal oxides from triblock copolymers and metal chlorides. Microporous and Mesoporous Materials, 118(1-3), 443-452.
- Drisko, G. L., Kimling, M. C., Scales, N., Ide, A., Sizgek, E., Caruso, R. A., et al. (2010). One-pot preparation and uranyl adsorption properties of hierarchically porous zirconium titanium oxide beads using phase separation processes to vary macropore morphology, Langmuir, 26(22), 17581-17588.
- Griffith, C. S., De Los Reyes, M., Scales, N., Hanna, J. V., & Luca, V. (2010). Hybrid inorganic−organic adsorbents Part 1: synthesis and characterisation of mesoporous zirconium titanate frameworks containing coordinating organic functionalities. ACS Applied Materials and Interfaces, 2(12), 3436-3446.
Published: 23/05/2011