The recent Super Bowl final may owe some kudos to polymer science and neutron scattering, which one of the world’s leading experts was on hand to explain why at a lecture hosted by ANSTO.
Transportation systems pay thanks to the development of advanced materials to heighten their performance.
The same can be said for sporting aficionados who tip their hats to a material science has only started to get its head around - polymers.
This was how Emeritus Professor Dame Julia Higgins, one of Europe’s leading experts started her history lesson on polymer science and scattering at a lecture series hosted by ANSTO.
 |
| This above animation illustrates examples of both common and advanced polymers used in everyday life. |
“Most of the pretty pictures I have here are in sports,” she said referring to a slide (illustrated to the right) that accompanied her presentation to scientists and guests on polymer science as part of the 20th edition of ANSTO’s Distinguished Lecture series and the first for 2012.
Polymers on the playing field
“Polymers are all around us. We think of them usually as commodity materials and they’ve been so for the last 50 or 60 years,” she said, but their application has become more refined as the knowledge of the science has evolved.
Strength, ductility, stiffness, temperature capability and the forgiveness that has been forged into many of the sporting items we take for granted is in part due to the application of polymer science and neutron scattering.
Neutron scattering is a technique that allows us to determine the properties of matter on the atomic level and then use the knowledge to optimise them or to develop new materials and functionality.
Grid iron players are a great example. They are an avid user of new and high-tech materials which are often the result of marrying neutron science with polymers.
According to a recent report in the New York Times, a 2000 study surveyed 1,090 former N.F.L. players and found more than 60 percent had suffered at least one concussion in their careers and 26 percent had had three or more.
With serious head injuries of major concern in the NFL, a lot of effort is placed into countering this trend, including developing modern body armour such as protective helmets to shield the players. This is where polymers come into play.
But what exactly are polymers? Having one of the early pioneers on hand to explain its history for an Australian audience helped shed light on the subject.
“I think everyone knows that they are long, covalently bonded molecules,” the now retired Professor Dame Julia explained to the audience made up mainly of scientists and peers.
To those who are not in the know, polymers are a long chain of small units of two or more monomers, which are molecules that can be bonded to other identical molecules.
There are natural polymers like cellulose and rubber which have been around since the dawn of time and synthetic polymers developed in labs such as plastic. A number of novel uses have been found for polymers that extend beyond the sporting field including synthetic heart valves in health through to most everyday domestic items you find around the house.
History of polymer science
In a nutshell, they are everywhere and are produced at such a rate that synthetic polymer production has now grown to be larger than the aluminium, copper and steel industries.
Historically, not a lot was known about polymers. The first modern example of the science was when sulphur was added to raw natural rubber in the 1840s helping to stop the material being sticky.
Professor Dame Julia said the field was helped by a radical shift in thought in the second half of the last century.
“When polymers became popular, which was really the Second World War there were two key scientists in this field. One was Herman Staudinger, a German scientist who was the first to really insist that these plastic materials that were around were actually covalently bonded linear molecules.
“Paul J Flory was the second key player who described the physical chemistry of these long molecules in a number of wonderful publications,” she said.
Dr Staudinger’s assumption that you could make hundreds or a thousand repeat units long for a polymer was developed in the 1920s and was considered impossible by most chemists at the time. It took years for his ideas to be accepted. He was awarded for his efforts receiving a Nobel Prize in 1953.
Fellow Nobel Laureate Dr Flory described how big the molecules would be, which at the time was difficult because no one was able to physically see a polymer in the melt, which is a polymers transition from a crystalline or semi-crystalline phase to a solid amorphous phase.
Flory’s prediction for the size of the molecule provoked big questions for scientists. If you have a very long molecule, what shape does it adopt? Does it collapse into a hard sphere; does it expand out into a long, wavy object, or what?
Another question was what would happen if you took an object like a rubber material and stretched it? And what happens when you let go? Will the deformed object go back to its original shape?
Polymers and neutron science
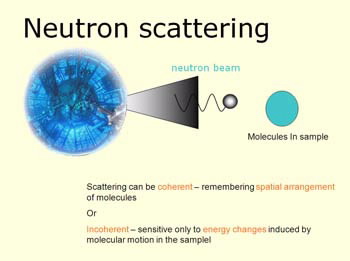 |
| An illustration of the neutron scattering process |
This is where Dame Julia work, along with her peers, comes to the fore. The Professor of Polymer Science joined the field as a doctoral student just as neutron scattering techniques were exploding on the scene as a tool to exploit the neutron.
She later co-authored a booked Polymers and Neutron Scattering with French scientist Henri Benoit which was specifically written to introduce the newcomer and non-expert in physics or chemistry to the experimental techniques and the basic theory necessary to understand the results.
With the ability to scatter free neutrons by matter scientists were able to explore many of the complex issues at the atomic level. This answered many questions, but opened a pandora’s box of equally complex problems.
“Chemists very early on noticed that the neutrons property of being scattered differently by hydrogen and deuterium offered a whole new set of physical chemistry type-problems that you wanted to label molecules or bit of molecules because the chemistry of putting deuterium where hydrogen was is not difficult and you could then you spectroscopic techniques to differentiate where the hydrogen was and the deuterium was.
“The main fact was neutrons are scattered incoherently mainly as a result of hydrogen but not so strongly by deuterium.
“Both the coherent and incoherent scattering were different. By replacing hydrogen with deuterium you had a marvellous labelling technique,” she said.
Today, small-angle neutron scattering is widely used in determining the size and shape of polymers and understanding their fundamental behaviour. The results have assisted many industries from those already mentioned to the aerospace industry and even down to everyday items used in homes.
The science continues to evolve and provoke thought among scientists, which the polymer expert says makes for a promising future for all involved.
Polymer facts: |
|
Published: 16/02/2012


