Nuclear scientists are a step closer to improving the detection of some of the most deadly skin cancers following successful human trials of a novel image tracer for PET scanners.
Australia has the world’s highest incidence rate for melanoma. Melanoma accounts for 10 per cent of all cancers in Australia. Malignant melanoma is a very aggressive cancer, with a high rate of metastasis brought about by excessive ultraviolet (UV) exposure. A key feature of melanoma is the extensive pigmentation present in most tumour cells.
Accordingly, this pigmentation, melanin, is a very attractive target for both disease localisation and treatment. As part of our radiopharmaceutical optimisation program we have developed a series of nicotinamide structures to target melanin with high selectivity.
Preclinical studies indicated that [18F]N-(2-(diethylamino)ethyl)-6-fluoronicotinamide ([18F]Mel050) was the most promising Positron Emission Tomography (PET) radiotracer for clinical staging with great potential for personalising therapeutical treatments. Preliminary patient studies appear to confirm these results.
Why we study melanoma
Skin cancer is the third most common human malignancy with 2-3 million new cases estimated across the world each year. Although melanoma accounts for only about 130,000 of these, it is the most dangerous form and results in most of the deaths related to skin cancer.
Australia has the world’s highest incidence rate for melanoma accounting for 10 per cent of all cancers - the fourth most common. Survival largely depends on early detection and cure by surgical resection of primary melanomas. Extensive metastatic malignant melanoma is refractory to most therapies with a median survival of 6 months and a 5 year survival rate of less than 5 per cent [1], although surgery may still be effective if metastases are localised.
Despite a paucity of effective treatments for advanced melanoma currently, improved diagnostic methods have considerably decreased mortality rates in early disease. Radiopharmaceuticals that can target the melanoma tumours may offer opportunities for better assessment of disease extent and thereby improve the selection of patients with metastatic disease for surgical resection, as well as potential radiotherapeutic applications [1 - refer to image library below this research].
A key feature of melanoma tumours is the extensive pigmentation present in most melanoma tumour cells, thus making melanin a very attractive target for both diagnosis and treatment.
To date, we and others have developed a considerable number of [123I]benzamide derivatives that exhibited good uptake in melanoma tissue and could be used for single photon emission computed tomography (SPECT) imaging and disease localisation.
Our attempts to improve the tumour to background ratios and body clearance of iodobenzamides led to the development of iodonicotinamide analogues [2] which are readily taken up in melanoma tumours with subsequent melanin binding.
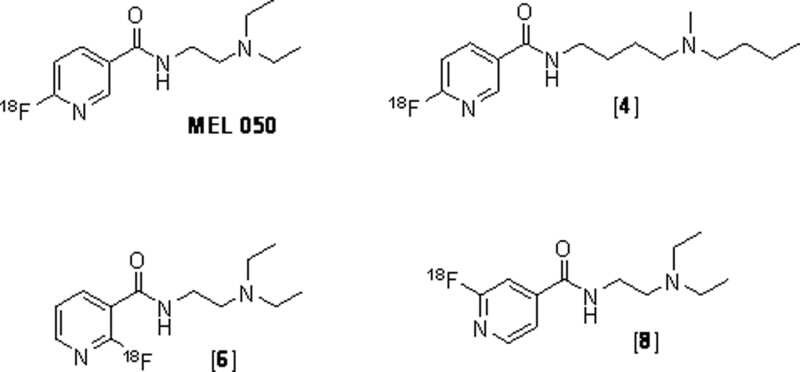 |
| Fig 1. The structures of fluoronicotinamide derivatives; [18F]N-(2-(diethyl amino)ethyl)-6 fluoronicotinamide ([F] MEL050), [18F]4, [18F]6, and [18F]8. |
Our approach
Positron emission tomography (PET) has emerged as a valuable imaging tool due to its ability to provide high resolution and absolute quantitative uptake in tissue, with ease in background subtraction versus the older SPECT imaging.
At present, 2-[18F]fluoro-2-deoxy-D-glucose [18F]FDG PET imaging of melanoma is the only PET clinical radiotracer used routinely to localise melanoma tumours [3].
Although [18F]FDG is an effective tool for melanoma tumour detection, inflammation and infection decrease its specificity [4] and partial volume-effects limit its sensitivity for small-volume disease.
Our objective was to improve specificity by incorporating fluorine-18F into the nicotinamide structure while retaining high melanin binding affinity and its rapid whole body clearance of unbound tracer, stability, and ease of radiolabelling.
We have developed a series of new PET [18F] fluoronicotinamide radiotracers that can be prepared in one simple radiosynthetic step [5-7]. One of them, [18F]N-(2-(diethylamino)-ethyl)-6-fluoronicotinamide ([18F]Mel050), displayed rapid clearance, superior in vivo stability, high tumour to background ratio in animal PET imaging and biodistribution studies and, in particular, high specificity for melanin.
Preliminary patient studies of [18F]Mel050 have demonstrated that the tracer is safe and can effectively demonstrate the location of normal melanin containing tissues and pigmented melanomas.
If this data is confirmed in larger patient studies, [18F]Mel050 could be a useful PET tracer for clinical staging and metabolic characterisation.
This should enable more accurate patient selection and planning of treatment as well as better therapeutic monitoring in melanoma compared to existing standards including [18F]FDG.
Making and testing the radiopharmaceutical The [18F]fluorine atom was added directly onto the nicotinic ring by direct [18F]fluorination of the chloronicotinamide precursor. Rapid radiosynthesis was achieved. This is critical since the 18F half-life is only 109 min. Various [18F]Fluoronicotinamides derivatives; [18F]Mel050, [18F]4, [18F]6, and [18F]8 (Fig. 1) were prepared to determine which had the best characteristics.
These radiotracers were ready to use within 40 minutes. This total process included purification and formulation from their chloronicotinamide precursors.
High radiochemical yields, radiochemical purity and specific activity were obtained. The radiochemical stability was maintained at >98 per cent over 3-4 hours in saline. The biodistribution of the [18F]fluoronicotinamides were studied in two mice strains: black mice bearing the murine melanotic melanoma and nude mice bearing the human amelanotic tumour.
A skin lesion (abnormal areas of tissue on the body) that is amelanotic, lacks the pigment melanin and therefore is essentially colourless. It would be expected that the fluoronicotinamides, if specific, would localise in the melanotic, but not in the amelanotic tumours.
Melanoma patients were recruited that had [18F]FDG positive lesions. The patients were then imaged with a GE PET/CT camera using [18F] [18F] Mel050 at the Peter MacCallum Cancer Centre that was prepared at Cyclotek, a commercial provider of radiopharmaceuticals.
The intense uptake of the tracer seen in the bladder confirms that MEL050 is cleared by the body mainly through the kidneys (patient’s left) and that there is only low uptake in important tissues such as the liver (patient’s right) and bone marrow, which are potential sites of secondary spread of melanoma.
Note: Red represents higher while green represents lower concentration of [18F]MEL050.
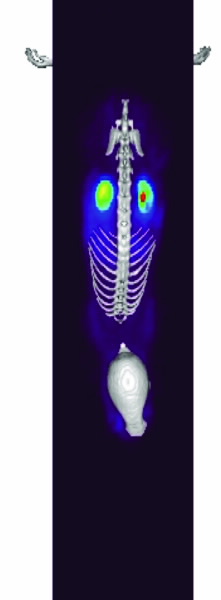 | 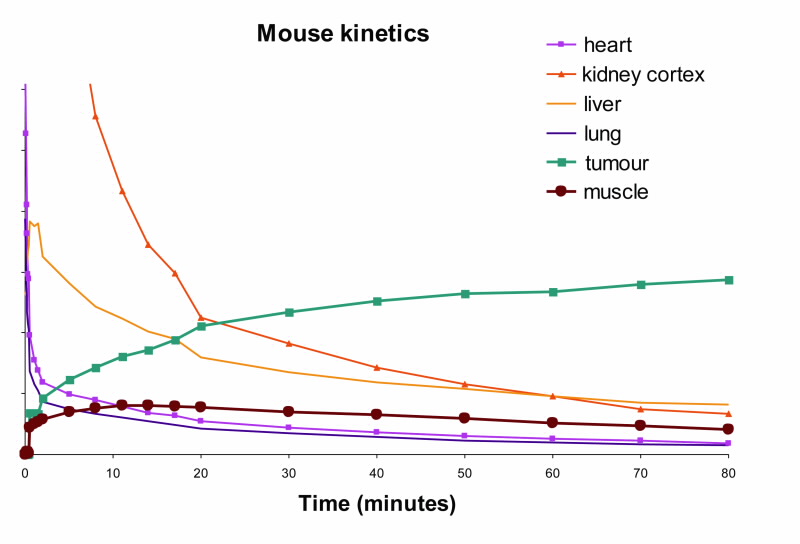 |  |
| Fig 2. [18F]Mel050 PET/CT image analysis of murine melanoma. (a) 2 minute whole body image (kidney and skeleton visible) of a C57BL/6J black mouse bearing a melanotic (B16F0) tumour (left flank), (b) graphical representation of uptake in a variety of tissues as a function of time demonstrating normal tissue washout and rapid tumour uptake (c) whole body images (tumour, stomach and eyes visible) at 80 minute PI. Note: Green represents low and red high radioactive concentration. |
A pilot study: from animal to humans
Table 1 shows the uptake of the different [18F] fluoronicotinamides as a function of time. Eye and tumour concentration for all analogues were relatively stable in this time frame where as the compounds rapidly washed out of the non-melanotic tissue.
Localisation in the eyes of mice was significant but similar to that of other melanin binding compounds [8,9].
Uptake values in the murine melanotic tumour and in the eyes of black mice at 3 h were more than 200 times greater than that observed in the nude mice bearing the amelanotic tumour. The large uptake differences between black and nude mice support the hypothesis that [18F]fluoronicotinamides are involved in a specific interaction with melanin.
High concentrations of melanin are present in the murine melanotic tumour and the pigmented eye structure of black mice, but are lacking in nude mice [2]. [18F]Mel050 was the best of the four compounds studied. It displayed the least bone uptake of all the derivatives and highest tumour to bone ratio at 0.25 and 3 hr PI (Table 1).
Bone uptake was comparable to that reported for a study using [18F]DAFBA [8].
Also, [18F]Mel050 was very stable in vivo. Analysis demonstrated that more than 90% of activity in tumour, eyes, and urine and 70% in plasma remained [18F]Mel050 for at least 2 h PI. The low bone uptake also suggested stability in rodents.
Fluorine cleaved from the molecule would be expected to lodge in the bone.
The rapid uptake and fast normal tissue washout of [18F]Mel050 is confirmed in the dynamic animal PET/CT image (Fig. 2). Initial images (Fig. 2b) demonstrated only renal uptake while later images show that only eyes, stomach, and tumour retain activity (Fig. 2c). Fig. 2a demonstrates that a high tumour to background ratio, ~10:1, was achieved at 80 min PI.
[18F]Mel050 was further evaluated in experimental mouse models of melanoma metastases in the lungs and in the lymph nodes (see Fig. 3).
A significant accumulation of the compound was shown in the lungs of mice bearing melanoma metastases.
The level of [18F]Mel050 signal in the images reflected the tumour burden with a strong link between [18F]Mel050 signal and the presence of melanoma deposits at necroscopy (6). [18F]Mel050 had also the ability to identify lymph node metastases when the tracer was injected intravenously
In addition, [18F]Mel050 allowed visualisation of nodal lesions below the theoretical spatial resolution of PET when administered locally into subcutaneous tissues around the primary lesion site (10).
All together, these results provide evidence that [18F]Mel050 has the potential for whole-body staging of pigmented melanoma as well as for the sensitive and specific identification of lymph node involvement when administered locally at the primary tumour site.
Because of this successful pre-clinical data, animal toxicology studies of Mel050 were performed as a prerequisite for human clinical studies.
The lack of observable toxicity in rats at doses of Mel050 one thousand times that proposed for human studies supported the initiation of first in human studies to evaluate safety, biodistribution, tracer stability and to obtain preliminary information about melanin targeting potential.
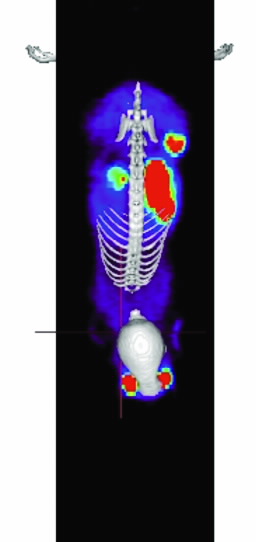
To date, five melanoma patients have been imaged in a pilot clinical study at the Peter MacCallum Cancer Centre supported by the Cooperative Research Centre for Biomedical Imaging Development (CRC-BID). Fig. 4 is an example of a patient image from this study and demonstrates the advantage of [18F]Mel050 compared to a CT scan.
Of these five, two patients had no [18F]Mel050 retention in their [18F]FDG-positive metastatic melanoma lesions.
Histological examination of tumour samples indicated an absence of melanin. In contrast, three patients with melanin detected at pathological tumour evaluation had high [18F]Mel050 uptake in their [18F]FDG-positive lesions.
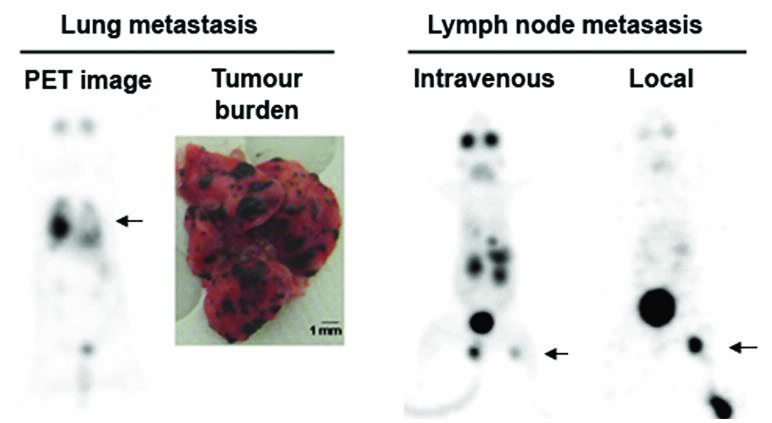 |
| Fig 3. Whole-body PET images of lung metastases obtained 2 hours after [18F] Mel050 injection and corresponding necropsy specimen (left). Lymph node metastases imaged either after intravenous or subcutaneous peri-lesional injection (local) of [18F]Mel050 (right). The black arrows indicate melanoma metastases. |
Next steps
It was somewhat surprising that our small patient study had a high percentage of patients with amelanotic tumours, however this finding may reflect the biology of the tumours at more advanced clinical stages. If identifying patients with amelanotic tumours had prognostic significance, i.e., had a poorer prognosis, [18F]Mel050 could provide additional critical staging information to the standard patient work-up. [18F] Mel050 could also be an effective means of following therapeutic efficacy of new therapies that are targeted to melanin.
Lastly, as indicated above, the survival rate for patients with metastatic melanoma is poor. Work has continued to develop a radiotherapeutic derivative of Mel050 to treat patients with melanoma. We are designing Mel050 analogues labelled with therapeutic radionuclides: 131I, 177Lu or 90Y to achieve favourable biodistribution characteristics.
This would be high tumour residence time (high radiation to the tumour) and low normal tissue uptake (low radiation to normal issue). These new treatments could provide hope to the patients afflicted with this disease.
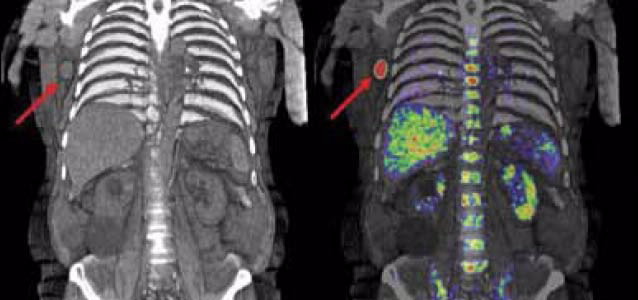 |
| These images show a patient who has had a scan using MEL050 to identify the spread of melanoma through the body via the lymphatic system. The CT image on the left identifies the location of the melanoma metastasis in an enlarged right axillary lymph node.The image on the right illustrates the improved diagnostic value that the combined PET/CT scan offers clinicians and patients through better definition of the tumour’s location, size and spread. The intense uptake of the tracer seen in the bladder confirms that MEL050 is cleared by the body mainly through the kidneys (patient’s left) and that there is only low uptake in important tissues such as the liver (patient’s right) and bone marrow, which are potential sites of secondary spread of melanoma. Note: Red represents higher while green represents lower concentration of [18F]MEL050. |
Table 1
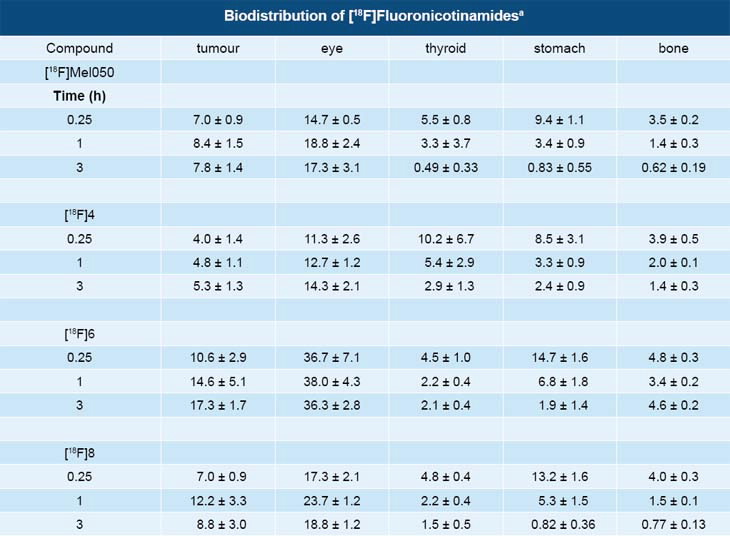 |
| Table 1 - aData are the means of %ID/g of tissue ±SD; n = 5. B16F0 melanoma tumour in C57BL/6J mice. |
Authors
Ivan Greguric1, Tien Pham1, Stephen Taylor1, Patrice Ballantyne1, Christian Loc’h1,Andrew Katsifis1, Rob Ware2, Marie Gregoire1, Delphine Denoyer2, Rodney J Hicks2, Ron Weiner1.
1ANSTO, 2Centre for Molecular Imaging, Peter MacCallum Cancer Centre, Melbourne
References
- Cummins, D. L., Cummins, J. M., Pantle, H., Silverman, M. A., Leonard, A. L., & Chanmugam, A. (2006). Cutaneous malignant melanoma. Mayo Clinic Proceedings, 81(4), 500-507.
- Liu, X., Pham, T. Q., Berghofer, P., Chapman, J., Greguric, I., Mitchell, P., et al. (2008). Synthesis and evaluation of novel radioiodinated nicotinamides for malignant melanoma. Nuclear Medicine and Biology, 35(7), 769-781.
- Belhocine, T. Z., Scott, A. M., Even-Sapir, E., Urbain, J. L., & Essner, R. (2006). Role of nuclear medicine in the management of cutaneous malignant melanoma. Journal of Nuclear Medicine, 47(6), 957-967.
- Hafner, J., Schmid, M. H., Kempf, W., Burg, G., Kunzi, E., Meuli-Simmen, C., et al. (2004). Baseline staging in cutaneous malignant melanoma. British Journal of Dermatology, 150(4), 677-686.
- Greguric, I., Taylor, S. R., Denoyer, D., Ballantyne, P., Berghofer, P., Roselt, P., et al. (2009). Discovery of [18F]N-(2-(diethylamino)ethyl)-6-fluoronicotinamide: A melanoma positron emission tomography imaging radiotracer with high tumour
- to body contrast ratio and rapid renal clearance. Journal of Medicinal Chemistry, 52(17), 5299-5302.
- Denoyer, D., Greguric, I., Roselt, P., Neels, O. C., Aide, N., Taylor, S. R., et al. (2009). High contrast PET imaging of melanoma using [18F]MEL050, a selective probe for melanin with predominantly renal clearance. Journal of Nuclear Medicine, 51(3), 441-447. Table 1
- Greguric, I., Pham, T. Q., Liu, X., & Katsifis, A. (2008). Nicotinamide Derivatives for imaging and therapy of melanoma. Patent No: 2008901989.
- Garg, S., Kothari, K., Thopate, S. R., Doke, A. K., & Garg, P. K. Design, synthesis, and preliminary in vitro and in vivo evaluation of N-(2-diethylaminoethyl)-4-[18F]fluorobenzamide ([18F]-DAFBA): a novel potential PET probe to image melanoma tumors. Bioconjugate Chemistry, 20(3), 583-590.
- Pham, T. Q., Berghofer, P., Liu, X., Greguric, I., Dikic, B., Ballantyne, P., et al. (2007). Preparation and biologic evaluation of a novel radioiodinated benzylpiperazine, 123I-MEL037, for malignant melanoma. Journal of Nuclear Medicine, 48(8), 1348-1356.
- Denoyer D., Potdevin T., Roselt P., Neels O.C., Kirby L., Greguric I., Katsifis A., Dorow D.S., Hicks R.J. (2011). Improved detection of regional melanoma metastasis using 18F-6-fluoro-N-[2-(diethylamino)ethyl] pyridine-3-carboxamide, a melanin-specific PET probe, by perilesional administration. Journal of Nuclear Medicine Jan;52(1):115-22.
Published: 27/04/2012


