It holds us together by way of a number of select variabilities. In this research project, the aim was to understand some of those traits arising around cell-behaviour at low temperatures and low water contents.
 |
| Figure 1. An illustration of cell membranes that separates te interior of all cells frm the outside environment. |
Introduction
Cell membranes exist as selective barriers between the cell cytoplasm, and various intracellular compartments and the extracellular world. Maintaining the correct functioning of this permeability barrier is a key issue in the viability of the cell after cooling and/or drying.
Our current work aims at understanding fundamental questions in membrane biophysics in the context of scientific problems arising around cell-membrane behaviour at low temperatures (cryo-biology) and at low water contents (anhydro-biology).
We looked into the effects of small solute molecules in terms of the stability of membranes and the redistribution of water and various sugars during both dry and cold environments.
Measurements resolving important intermolecular distances, have been used to build a theoretical model for effects of sugars on the rearrangement of lipids during dehydration and the consequential loss of membrane integrity under these conditions.
The effects of drying and freezing on membranes
In slow drying conditions, as is normally found in the natural environment, and at temperatures above the formation of a glassy state, molecular mobility comes to a halt. Small solute molecules, typically sugars, are used by organisms which live and reproduce in very cold and dry environments to protect their membranes from these deleterious phase transitions [1].
In this case one can assume that water potentials in a solution have come to equilibrium and solutes (substances dissolved in water) do not redistribute across the membrane appreciably during the drying process.
The effects of slow cooling are equivalent to slow drying. When ice forms in the extracellular solution the concentration of extracellular solutes is increased, and because the membrane is quite permeable to water, water may be drawn out of the cell much more quickly than solutes are transported in.
As further freezing occurs, more water is drawn out of the cell. Thus the net effect of freezing on slow timescales is to dehydrate and contract the volume of the intracellular solution inside the cell and is in fact similar to the effects of drying. The most evident impact of this are changes in the way lipids are packed into bilayers and other structures.
Lipids are the major structural components of cell membranes but also represent a broad group of important signalling molecules for biological functions such as energy storage. Fig. 1 depicts these effects and subsequent interactions.
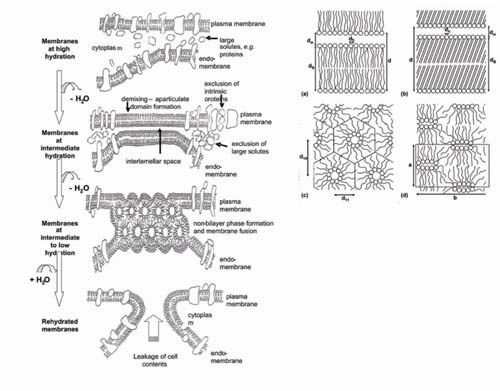 |
| Figure 2. Diagram of the effects of slow drying and cooling on the cell membrane |
Figure 2.
The left-hand side shows a diagram of the effects of slow drying and cooling on the cell membrane. The generation of compressive forces in the plane of the membrane by inter-membrane interactions, and subsequent phase transitions in the membrane lead to the loss of membrane integrity and functionality.
The dimensions of cells are 1/10’s to micron size. The important length scales shown in the right-hand side, the lipids phases associated with freezing or desiccation induced cellular damage, are of the order of nanometers for a single bilayer:
a) the fluid lamella phase is associated with normal functioning and consists of alternating layers of lipid bilayers (thickness, db) and water (thickness, dw) and separation between head groups, dh. In the fluid lamella phase the tail chains are packed rather randomly in the hydrophobic phase;
b) the gel lamella phase is very similar in geometry with the difference being a closer packing of head groups and a more ordered lipid phase [1];
c) the hexagonal phase causes loss of continuity of the lipid membrane and is characterised by a hexagonal symmetry with two characteristic repeat distances. Each hexagon has at its centre a circular channel projecting out of the page surface; and the ribbon phase where the unit cell is again characterised by two characterised by repeat distances [2];
d) A ribbon-like channel is formed by lipid head-groups and projects out of the page [3].
Quantifying these effects with X-ray and neutron scattering
Scattering techniques, particularly X-rays and neutrons, can be used as diagnostic of the phase of the lipids providing a method for measuring the distances shown in the right-hand side of Fig. 1.
In Fig. 2, we show X-ray measurements from which we can extract structural details relating to the shape and spatial relationships between coexisting phases, particularly when deuteration and contrast variation are applied [2-6].
Two very important structural parameters easily calculated from the position of diffraction peaks,see Fig. 2c, are: (i) the average chain-chain lateral spacing which can be used to estimate the average head-group spacing dh – see Fig. 1a, and (ii) the bilayer repeat spacing d, which can be used to estimate dw and db [1].
In addition, we used small-angle neutron scattering (SANS) with contrast variation (neutrons can distinguish between different elements and their isotopes) to investigate the partitioning of sugar molecules between the water between bilayers and the channels in the hexagonal phase (Fig. 1a) and a bulk aqueous phase which is not shown in the figure [5,6].
Fig. 2 shows how the scattering data can be interpreted to obtain the important information on the spacing in the lipid phases. Based on the distances measured and the partitioning of sugar we have developed a detailed and successful theoretical model to explain the effects of sugars in the bilayer system [2].
 |
| Figure 3. X-ray Scattering patterns from the area detector of a pin-hole SAXS camera (ID15 beam line, Advanced Photon Source |
Figure 3.
X-ray Scattering patterns from the area detector of a pin-hole SAXS camera (ID15 beam line, Advanced Photon Source, Argonne, USA) from dipalmitoylphosphatidylcholine:
a) in the fluid phase at 70°C and
b) in the gel phase at 20°C, both are the results of 10 second exposures.
The pin-hole scattering data is radially symmetric and together with the experimental geometry and wavelength of the scattered radiation we could produce the radial average shown in c).
The positions of the peaks are used to calculate the spacing of the inter-lamellar and lipid-packing shown in Figure 1 under conditions of dehydration and cooling, where cooling causes a more efficient packing of lipids and shift of peaks to the right.
Next steps
We intend to further our understanding of physical changes in membranes, particularly lipid packing, in anhydro and cryo-biology by looking at the role of lateral phase separation (lipid rafts). With the ability to deuterate various biomolecules at the National Deuteration Facility combined with neutron-scattering techniques, we particularly develop techniques for studying large unit-cells (cf Fig. 1) at higher resolution than is available with conventional small-angle neutron scattering.
In addition, we study lateral order in oriented systems such as bilayers by grazing-incidence scattering and off-specular reflectivity techniques.
Authors
Christopher J. Garvey1, Thomas Lenné 2,3, Ben Kent 1,3, Taavi Hunt 3 and Gary Bryant3
1ANSTO, 2ANU Canberra, 3RMIT Melbourne
References
- Andersen, H. D., Wang, C., Arleth, L., Peters, G. H., & Westh, P. (2011). Reconciliation of opposing views on membrane–sugar interactions. Proceedings of the National Academy of Sciences (PNAS), 108(5), 1874-1878.
- Lenné, T., Garvey, C. J., Koster, K. L., & Bryant, G. (2009). Effects of sugars on lipid bilayers during dehydration - SAXS/WAXS measurements and quantitative model. Journal of Physical Chemistry B, 113(8), 2486–2491.
- Kent, B., Garvey, C. J., Lenné, T., Porcar, L., Garamus, V. M., & Bryant, G. (2010). Measurement of glucose exclusion from the fully hydrated DOPE inverse hexagonal phase. Soft Matter, 6(6), 1197-1202.
- Kent, B., Garvey, C. J., Cookson, D., & Bryant, G. (2009). The inverse hexagonal – inverse ribbon – lamellar gel phase transition sequence in low hydration DOPC:DOPE phospholipid mixtures. Chemistry and Physics of Lipids, 157(1), 56-60.
- Lenné, T., Bryant, G., Garvey, C. J., Keiderling, U., & Koster, K. L. (2006). Location of sugars in multilamellar membranes at low hydration. Physica B: Condensed Matter, 385-386(2), 862-864.
- Lenné, T., Garvey, C. J., Koster, K. L., & Bryant, G. (2010). Kinetics of the lamellar gel–fluid transition in phosphatidylcholine membranes in the presence of sugars. Chemistry and Physics of Lipids, 163(2), 236-242.
Published: 16/08/2011


