An ANSTO researcher has co-authored a novel theoretical approach to explain inconsistencies between crystallographic and chemical experimental data in the apparent transformation of a pyrochlore to defect fluorite in La2Zr2O7.
The model can be extended to understand the ageing of a large class of complex oxides, such as spinels, with practical applications ranging from solid oxide fuel cells to the design and management of nuclear waste forms.
The research was published in Scientific Reports.
Prof. Gordon Thorogood, a nuclear fuel cycle researcher, who collaborated with colleagues including David Simeone from CEA and Da Huo a PhD student and others said what was thought to be a phase change to defect fluorite structure, may, in fact, not be occurring.
“Matrix mathematics suggested that a summation of peaks in the crystallographic data will make it look like the peaks have disappeared,” said Thorogood.
The diffraction patters of La2Zr2O7 are due to the interference of scattering waves between shifted pyrochlore nano-domains.
“The possibility has been viewed with surprise but considerable interest as the physical mechanism responsible for the formation of a defect fluorite structure remains unclear.”
In the investigation, they looked for a change in local symmetry that should have occurred with respect to decreasing grain size. Fine grained pyrochlore grains were sintered at different temperatures to probe order at different length scales.
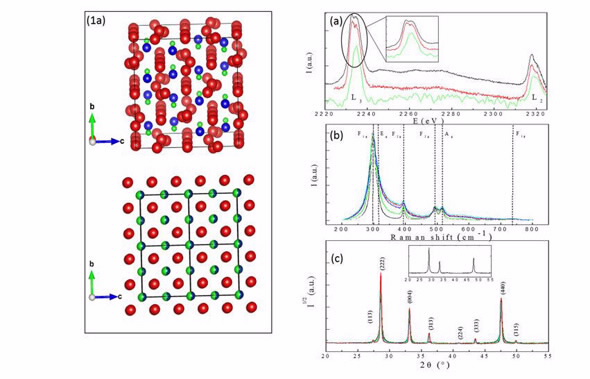 |
| Description of the structures (1a), EELS (a), Raman (b) spectra and X-ray diffraction patterns (c) collected on the La2Zr2O7 powders. |
Comparison of electron energy loss spectroscopy (EELS) spectra collected near the lanthanum edge of the zirconium indicated local symmetry in the zirconium did not vary with respect to grain size. Their results showed the valence state of the zirconium did not vary in small grain pyrochlores.
From group theory calculations, they determined the defect fluorite can be understood to result from an ensemble average of different perfectly ordered domains of specific size.
The defect fluorite structure is achieved without invoking any simultaneous disordering of anions and cations at the atomic level and the result of intricate disorder due to a random distribution of fully ordered nano-domains and it removes the need for a new phase at the atomic scale.
Defect fluorite is similar to the mineral fluorite with a single cation and anion site. The random mixing of oxygen and vacancies on a single site reduce the pyrochlore unit cell.
Understanding how damage occurs in a fluorite stricture is of great interest in the nuclear fuel cycle, because fluorite is the stricture of uranium dioxide (UO2), the most common nuclear fuel.
Radiation damage is thought to cause the change from pyrochlore to defect fluorite structure.
“With a phase change from pyrochlore to defect fluorite, you also have a volume change. But if that is not occurring, you need to understand why,” said Thorogood.
We would like to prevent swelling caused by damage to the fluorite matrices in nuclear fuel.”
“Now that we have determined the position of the oxygen, we can move to radiation induced damage studies."
http://dx.doi.org/10.1038/s41598-017-02787-w
Published: 05/09/2017


