The complementary power of combining multiple X-ray techniques to understand the unusual properties of hyperaccumulator plants has been highlighted in a new cover article just published in New Phytologist.
 |
X-ray fluorescence microscopy (XFM) at the Australian Synchrotron has been used by a consortium of international researchers led by Dr Antony van der Ent of the Centre for Mined Land Rehabilitation at The University of Queensland, in association with A/Prof Peter Kopittke of the School of Agriculture and Food Science also at The University of Queensland.
The XFM technique generates elemental maps showing where elements of interest are found within plant tissue, seedlings or individual cells.
Visually striking images (obtained at the XFM beamline) show various hyperaccumulator plants, on the cover of the April issue of New Phytologist. In the images each element is depicted in a different colour, making up a red-green-blue (RGB) image.
“Hyperaccumulators are of scientific interest because whilst metals are normally toxic to plants even at low concentrations, these plants are able to accumulate large concentrations without any toxic effects,” he added“Hyperaccumulator plants have the unusual ability to accumulate extreme concentrations of metals and metalloids in their living tissues,” said van der Ent.
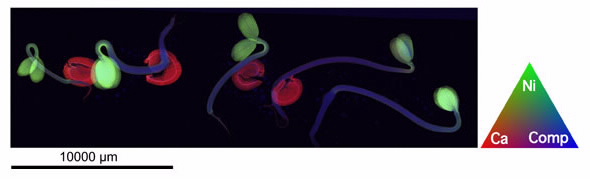 |
| Elemental map of live seedlings of the nickel hyperaccumulator Alyssum murale (Brassicaceae) obtained by X-ray fluorescence microscopy |
“The question is ‘how are they able to do that and what is the mechanism which enables them to tolerate such high metal concentrations?’ Is there something we can learn from in terms of trying to understand metal toxicity in normal plants— particularly in those we use in agriculture?”
“Studies of the hyperaccumulators are also undertaken for applications in phytomining (a novel approach to involves cultivating hyperaccumulator plants as ‘metal crops’ to produce valuable metals),” said van der Ent.
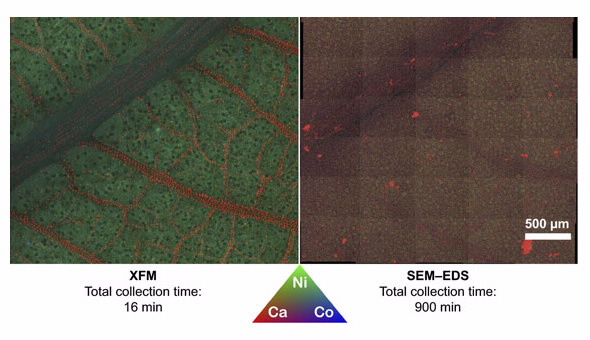 |
| Elemental distribution maps (Ni, Co, Ca) obtained with X‐ray fluorescence microscopy (XFM) (left) and scanning electron microscope (SEM) (right) of an identical sample (Rinorea javanica leaf). |
Elemental distribution maps (Ni, Co, Ca) obtained with X‐ray fluorescence microscopy (XFM) (left) and scanning electron microscope (SEM) (right) of an identical sample (Rinorea javanica leaf).
XFM beamline scientist Dr Martin de Jonge, a co-author on the paper with Dr David Paterson, said the technique was used to study how metals are taken up in roots and distributed throughout the plant under ‘lifelike’ conditions.
Among the different X-ray techniques that were evaluated in the paper, each has its own advantages. Kopittke said he was a strong proponent of XFM for this very reason.
“Because a vacuum is not required, you can undertake the experiment in ambient conditions. Plants are more than 90 per cent water and if you have to dehydrate them to put them in a vacuum as you do for other X-ray techniques, you invariably move the elements you are analysing. With XFM we can analyse a live plant in its natural state, and this is highly advantageous. You cannot do this with any other technique, “said Kopittke.
The authors note that as XFM can probe to a depth from several hundred micrometres to the millimetre scale, it is ideal for addressing plant science. “Its high sensitivity and capacity to be used on large samples make it very versatile,” said de Jonge.
Unique to the XFM beamline is the use of a revolutionary X-ray detector Maia which is an extremely fast detector system. Maia makes it possible to obtain elemental maps that are many millions of pixels (megapixels) in size in hours. Because of this, there was no visible evidence of radiation-induced damage to live plants.
XFM can simultaneously map hyperaccumulated metals (such as nickel), important nutritional elements (such as potassium) and trace elements that are typically present at far lower concentrations (such as copper).
“You can illuminate with a single energy and you get the fluorescence from everything, known as the spectral axis,” said de Jonge. “But you can also modify the incident energy and look at changes in the spectral axis as a result of that.This helps you probe the electronic structure of atoms.“
The other techniques that were comprehensively reviewed in the paper included proton induced X-ray emission (PIXE). This technique can be undertaken on frozen samples; thereby making it possible to investigate cross-sections of plant tissue to elucidate the internal cellular-level distribution of elements. PIXE is also ideally suited to measure very light elements, such as aluminium.
“The complementarity of X-ray elemental mapping techniques provides a way to answering questions at every level about how plants take up metals and maintain their physiological functions” said Kopittke.
Collaborating institutions included The University of Queensland, University of Adelaide, Université de Lorraine (France), iTHEMBA Labs (South Africa), University of Science and Technology (Poland), Jagiellonian University (Poland), and CSIRO.
https://doi.org/10.1111/nph.14810
Published: 05/04/2018


