An international collaboration between human health researchers at ANSTO, the Centre for Medical Radiation Physics at the University of Wollongong and the National Institute of Radiological Sciences (NIRS), Japan, has undertaken research relating to carbon ion therapy, an advanced form of cancer treatment being proposed for introduction in Australia.
The study investigated the use of a beam of positron-emitting radionuclides for the precise delivery of non-invasive and highly conformal radiotherapy.
The radioactive beam matched the therapeutic performance of current non-radioactive heavy ion beams, but offers a greatly improved ability to verify the delivered dose distribution during treatment, through the use of in-beam PET imaging.
“The mechanism provides a potential way to experimentally verify that the actual delivered dose is the same as the treatment plan in real time” said co-author Dr Mitra Safavi-Naeini, Imaging Quantification Research Lead, Human Health at ANSTO.
The research has been submitted and is under review for publication in Physics in Medicine and Biology.
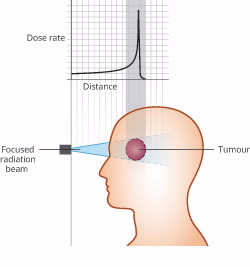
Cancer treatment with heavy ions
Particle therapy using heavy ions such as carbon delivers a highly targeted peak dose of therapeutic radiation at a specific depth that coincides with the treatment region.
Most of the energy is deposited within a very narrow depth range, minimising damage to surrounding healthy tissue.
 |
“Because of the intensity of the dose, treatment approaches tend to be very conservative,” said Safavi-Naeini .
“The research was exploring enhanced quality control measures for treatment delivery verification.”
“Because a large proportion of positron fragments produced in collisions with the nuclei of target cells stop very close to where the maximum dose is delivered, you have a means of verifying the site of deposit using PET,” said Safavi-Naeini.|
The study compared the use of stable ions and radioactive ions to produce positrons for PET imaging and found the radioactive beam greatly outperformed the stable beam.
A comparison was undertaken using ions at different energy levels in several phantoms (simulated human tissues and organs).
The radioactive ion beam was found to have a relative biological effectiveness (RBE) within 4% of the stable beam, showing that it was therapeutically equivalent.
“If you can deliver a radioactive beam with the correct fluence, the appropriate number of particles at the right energy to the target, then you should be able to use it therapeutically,” said Safavi-Naeini.
Enhanced PET imaging
Using a beam of positron-emitting radionuclides has advantages over a stable ion beam, as it enhances the signal and provides better visualisation of the deposited dose distribution.
While most of the heavy ions in the beam stop and deposit their kinetic energy close to the planned target depth (at the site of the tumour) without undergoing a nuclear interaction, a fraction will collide with nuclei in the target, producing a range of nuclear fragments.
Some of these fragments are unstable, and decay via positron emission over the next few seconds to minutes. These positrons annihilate with electrons in the target and produce pairs of gamma rays, which can be imaged during and immediately after treatment.
The use of a beam of ions which are, by themselves, short-lived positron-emitting radionuclides greatly increases the number of positrons emitted in the target region, leading to a better-quality image.
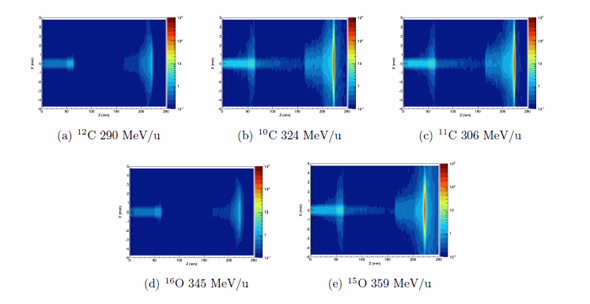 |
| Spatial distribution of positron creation in a lung phantom, for each beam type |
The study included generation of experimental and simulated 3D and 4D PET images of positron yields for multiple ion types and energies, and an estimate of the corresponding relative biological effectiveness for each.
The investigators used simulations to calculate the dose deposited as a function of depth, utilising advanced microdosimetric modelling techniques to evaluate the relative biological effectiveness of each beam and predict the yield of positrons in targets.
Experimental validation of the simulation model for depth-dose profiles was performed using the Heavy Ion Medical Accelerator in Chiba (HIMAC), Japan.
The simulation reported only a 0.8 mm difference in the location of maximum dose compared with experimental data.
“The approach could reduce the time needed for post-treatment PET imaging while improving the accuracy of the result, both leading to better patient outcomes” said Safavi-Naeini.
ANSTO is supporting the introduction of a National Particle Therapy and Research Centre in Australia in association with leading hospitals, research centres, universities and industry.
There are more than 70 particle therapy facilities worldwide, and another 41 under construction. A proton therapy centre will be built in Adelaide with Federal and State funding.
Published: 17/05/2017


