From geohazard to future energy resource. ANSTO's research into gas-hydrates could help the oil and gas industries exploit these ice-lattice structures for their natural gas deposits and limit their impact as large solid plugs in underwater pipelines.
Introduction
Gas-hydrates are found in nature, in deep ocean sediments and in permafrost, but are not stable at atmospheric temperature and pressure. They are also found in marine oil/gas pipelines on the ocean floor, where they can agglomerate into large solid plugs which stop the flow of fuel, and consequently are see as a major risk for oil/gas industry exploitation.
Due to a lack of reliable information on the formation and dissociation of gas-hydrates, our understanding of the opportunities and risks presented by these compounds is limited.
As neutrons are very sensitive to the structure and dynamics of hydrogenous media, we have embarked on an investigation of the kinetic processes of gas hydrates using the neutron-beam instruments at the OPAL research reactor.
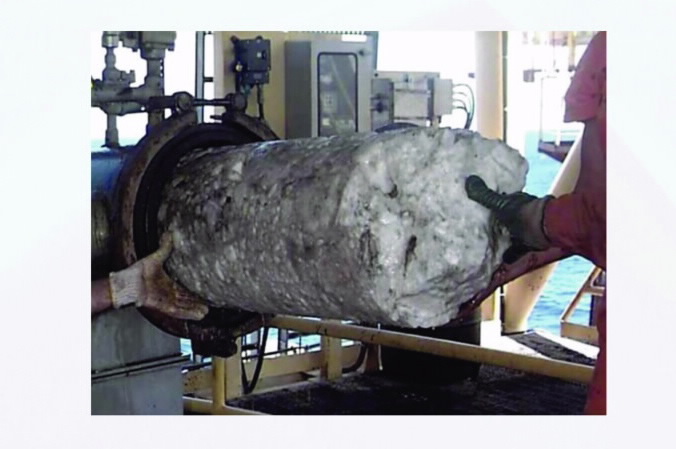 |
| Fig 1. Gas-hydrate shot plug being removed from a blocked pipeline. |
The impact of gas-hydrates
Gas-hydrates (or clathrate hydrates) are inclusion compounds, which form at low temperature and elevated pressure from the action of water molecules encapsulating gases of suitable size and shape (e.g. methane, propane or carbon dioxide).
Naturally occurring gas hydrates, containing mostly methane, are of particular interest in the energy sector as a potential future energy source, and environmental science as a geo-hazard.
Gas-hydrates pose a major risk of disruption to the marine oil and gas pipelines, see Fig. 1 (right), through blockages and breakages. Due to a lack of understanding of hydrate formation and dissociation processes, the gas mining industry currently takes extreme measures to reduce the risk of hydrate formation.
A clear understanding of these processes would allow implementation of effective strategies to avoid blockages in gas pipelines alleviating both economic and environmental concerns [1].
Neutrons scatter rather strongly from light elements like hydrogen and therefore they are a unique technique for investigating hydrates. In addition, they are a non-destructive probe for the study of the structure and dynamics of hydrogenous media at the atomic and molecular scale.
Consequently, ANSTO has initiated a comprehensive study of the kinetics of formation and dissociation of pure methane and methane-propane hydrates.
This joint research project with CSIRO aims to investigate the processes of formation and dissociation of gas-hydrates using the neutron-beam instruments at OPAL. Within that study we are comparing the effect of a range of known and potential inhibitors of gas hydrate formation processes using neutron diffraction and small-angle neutron scattering (SANS).
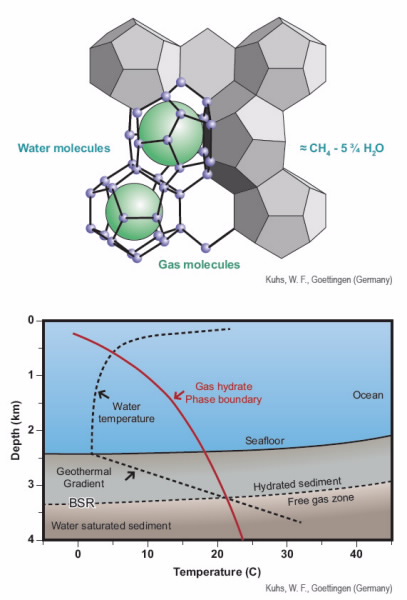 |
| Fig 2. (Top) gas hydrate, where a methane guest molecule is caged by water molecules, and (bottom) chart showing the stability of gas-hydrate beneath the seafloor at mid-latitudes. |
Hydrate formation
Fig. 2 (see image library below) shows one of the known clathrate hydrate crystal structures with a methane guest molecule. There are several known hydrate structures stemming from different mixtures of cages that form around hydrocarbons of differing size and shape.
With increasing water depth, the temperature of the ocean decreases and the water pressure increases.
Beyond depths of a few hundred meters, where the temperature drops to 4°C, the risk of gas hydrate formation in pipelines increases rapidly with depth (Fig. 2, bottom).
However, the probability of hydrate formation also depends on other factors, such as turbulence, or catalytic agents such as salts or other molecules that may be present in the gas or oil stream; hence posing a great challenge in view of replicating marine conditions.
Hydrate formation at the interface between water and hydrocarbon gases is quite rapid, but rates of gaseous diffusion into water are quite slow, and into solids are even slower. This means that, in the absence of turbulence gas hydrates only form thin layers (some microns in depth) on the surface of water droplets.
In the laboratory setting this means that gas hydrate will not form from bulk liquid water. Therefore we have seeded the hydrate formation process in the laboratory by starting with fine-ground ice crystallites.
This forms hydrate crusts on the fine ice particles from which we can further study the formation process at the liquid-water interface by melting the remaining ice cores.
Thus, we have been able to perform our experiments close to sub-sea geological conditions on the high-intensity neutron powder diffractometer Wombat, and the small-angle neutron scattering (SANS) instrument, Quokka.
ANSTO’s Wombat diffractometer used for the kinetic study of gas hydrates.
Experiments on Wombat
Our in situ experiments on Wombat provide insights into the kinetics of hydrate crystallite formation and dissociation, elucidating the thermodynamics of the reaction processes [2].
|
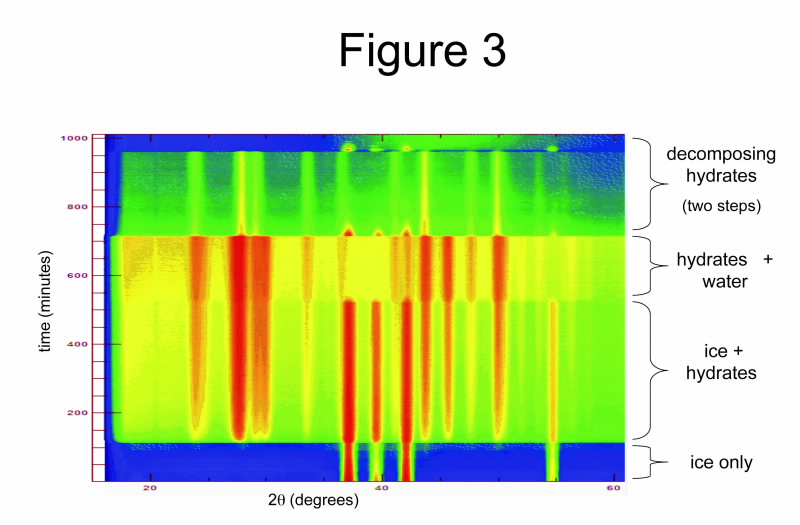
| Fig 3. Selected region of the Wombat diffraction patterns from hydrate formation by heavy water (D2O) in methane-propane gas at pressures up to 49 bar. |
Fig. 3, which is a colour-contour map of the neutron diffraction patterns in a reaction between heavy water (D2O) and high-pressure methane-propane gas, illustrates the formation process we observe.
|
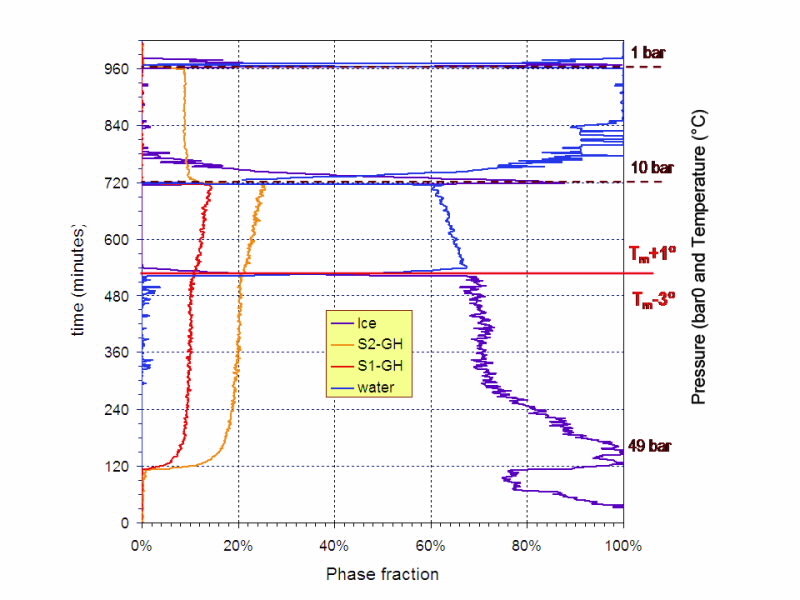
| Fig 4. Kinetics phase map of Ice, Type I & Type II hydrates and water, extracted from the neutron diffraction patterns of Figure 3. |
Note D2O is used instead of normal water due to its stronger diffraction signal, which leads to a higher sensitivity. In the figure, the stronger diffraction peaks are shown in red, and the green background indicates the presence on methane-propane gas at high pressure (49 bar).
Here, as we modify the temperature and pressure of the system, the diffraction reveals the kinetics of crystallisation of gas-hydrates; initially on the surface of ice crystallites and later from the interface between water and gas.
Finally, we see the dissociation of gas-hydrate on depressurisation, with a concomitant reformation of ice crystallites due to local cooling. In this example both hydrate structures Type I and Type II methane-propane hydrates form.
Fig. 4 displays the kinetics of the phase transformations that were extracted from the diffraction patterns shown in Fig. 3. In our neutron diffraction studies on Wombat, we have systematically varied temperature and pressure in a range of experiments to facilitate a mapping of dependencies of the chemical processes involved.
We observe a systematic increase in the rate of change of hydrate phase (either formation or dissociation) as we move away from the hydrate-H2O phase boundary. We also note that the kinetics are more sensitive to temperature changes than to pressure changes. These observations hold for both Type I and Type II methane-propane hydrates.
Additional information on the role and efficacy of a thermodynamic inhibitor, mono-ethylene-glycol (MEG), and a kinetic inhibitor (Luvicap EG, BASF Corporation) were observed.
We note that the effect of the addition of MEG is in proportion to the shift in water-ice phase boundary, so that large concentrations (~ 10%) are needed to produce a noticeable change. In contrast, the kinetic inhibitor is more effective, such that even 0.5% of Luvicap EG added to the water, will retard the growth of crystallites from the liquid gas mixture.
This is particularly noticeable for the Type I gas-hydrate phase.
Experiments on Quokka
Quokka is designed to detect structures and morphologies in the nanometre regime through SANS.
With in situ SANS experiments we aim to probe the hydrate nucleation process, which precedes the crystallisation processes observed in diffraction experiments.
Our first SANS experiment on Quokka revealed rapid uptake of methane gas on ice crystallites well below the accepted phase boundary, offering the prospect of valuable insights in planned future experiments using a gas-liquid flow loop.
Initially, the SANS maps the rate of growth of hydrate on ice crystallites, and later the increase in growth rate on melting of the ice cores. Fig. 5 shows the changes in SANS pattern during methane hydrate formation (Fig. 5, left) and dissociation (Fig. 5, right).
In Fig. 5 on the left where the temperature is ~ 4°C below ice point for heavy water, the slope of SANS decreases from Q-3.7 (grey dotted line) at 1 bar gas pressure to Q-3.1 (blue dotted line) after 6 hrs at 27 bar and 1 hr at 91 bar; indicating formation and thickening of a crust of methane hydrate on near-spherical ice particles.
In Fig. 5 on the right where the temperature is ~ 2°C above ice point for heavy water, the slope decreases further to Q-2.9 (green dotted line) after ~2 hr at 91 bar; indicating further thickening of the methane hydrate crust.
On depressurisation to 12 bar the methane hydrate slowly decomposes releasing methane gas to form water.
This provides an independent measure of the growth rate of methane hydrate and thereby on the diffusion rates of hydrocarbon gases through solid & liquid water and through solid hydrate material.
Future direction for our research
Our experiments so far enable us to differentiate between the dependencies of hydrate formation and dissociation on temperature and pressure, to gauge the effect of different hydrate inhibitors, and able to identify the differences in behaviour of Type I and Type II hydrates.
Thus, we have established that this method can be used to evaluate potential new kinetic inhibitors.
Our major challenge remains to be able to initiate gas-hydrate nucleation and growth from the liquid-gas interface under turbulent conditions (as is experienced in the marine environment) in a manner accessible by neutron beams.
Currently, we are developing a gas-hydrate flow loop specifically for neutron-beam studies in order to replicate industry conditions, and thereby to probe hydrate nucleation processes at the atomic and molecular scale, a goal which has so far eluded scientists working with other techniques.
Authors
Shane Kennedy1, Alice Klapproth1,2 and Ross Piltz1
1ANSTO, 2CSIRO
References
- Sloan, E. D., Koh C. A. (2008). Clathrate Hydrates of Natural Gases, Third Edition by Taylor & Francis Group, LLC.
- A. Klapproth, R.O. Piltz, S. J. Kennedy, V. K. Peterson, K. A. Kozielski, P. G. Hartley, Gas hydrate formation and decomposition close to subsea geological conditions, Proceedings of the 12th International Conference on the Physics and Chemistry of Ice (Sapporo, September 2010), edited by Yoshinori Furukawa, Gen Sazaki, Tsutomu Uchida, and Naoki Watanabe, Hokkaido University Press, 2011, Sapporo, ISBN978-4-8329-0361-6.
Published: 23/10/2011