During National Pain Week we take a close look at how the science of crystallography is used in drug development for chronic pain. 
Serving as a powerful analytical tool, crystallography allows us to peer inside a material and discover how the atoms that make it up are arranged.
Since the discovery of this technique 100 years ago it has been used everywhere from helping to improve materials used in aircraft, to analysing artworks and these days is commonly used to discover new drugs such as the ones used to treat chronic pain.
We’ve all experienced the feeling of accidently cutting our finger, burning ourselves on the hot stove or jamming our finger in the door. We simply call this ‘pain’ and have all felt it, but scientifically speaking there are a few things happening to cause that response.
The feeling of pain is due to the action of sodium channels sitting in the membranes of our nerves. Voltage-gated sodium channels (Nav) are essential for creating the electrical impulses that transmit signals throughout our nervous system.
Pain is not always a bad thing. It can act as a warning signal as it quickly reminds us to remove our hand from the fire to prevent further damage.
But for some people the pain never goes away. This type of pain is known as chronic pain. Painkillers can become ineffective for those who experience chronic pain, decreasing the options available for pain relief.
We know that when we have a local anaesthetic our sodium channels are temporarily blocked. This information has led crystallographers to investigate sodium channels for new analgesic drug development.
This research will allow scientists to develop new painkillers for people with chronic pain without the side effects of addiction and drowsiness.
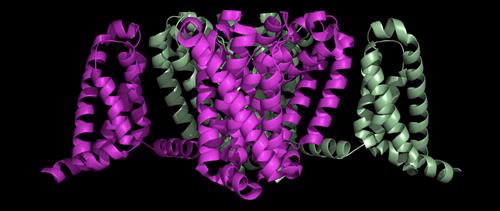
Structure of the Voltage-Gated Sodium Channel
What is it?
Humans have nine different sodium channels (Nav1.1 to Nav1.9). The Nav1.7 sodium channel is the one that plays a key role in pain transmission.
This was identified when researchers found that people with mutations in the Nav1.7 sodium channel did not feel pain (termed congenital indifference to pain).
The structure of a sodium channel in humans has not yet been determined but scientists have gained a lot of information from the structure of a bacterial sodium-selective channel.
What does it look like?
This is a side view of the bacterial sodium channel that sits in the membrane of the nerve cell:
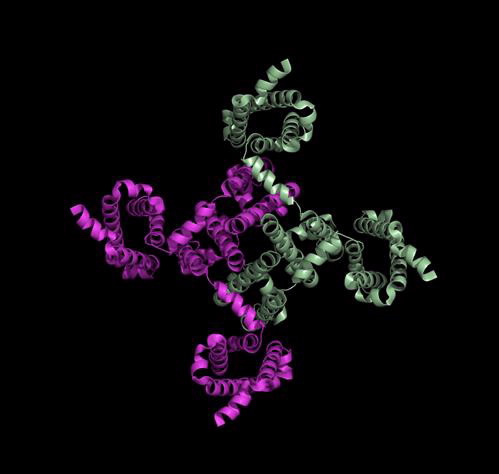
How do we use this structure to find more effective pain treatments?
Researchers are investigating toxins from venomous animals such as snakes, spiders, cone shells and plants as a source of painkilling molecules. This is because venoms have evolved over millions of years to target sodium channels, as this is the fastest way to incapacitate their prey.
Researchers from Australia have found a number of new molecules that are potential analgesics from these venoms. For example, they have found that venom used by a centipede to paralyse its prey, contains a molecule more effective than morphine.
This molecule is highly selective for the Nav1.7 channel over the eight other sodium channels found in the human body. The other sodium channels in the body, besides Nav1.7, play important roles in both heart and muscle function. If these other channels were to be blocked by a painkiller, we would get a number of unwanted side effects, including heart problems.
This makes finding a selective Nav1.7 channel blocker, such as the centipede venom molecule, an exciting step towards pain relief.
Where did the structure come from?
The crystal structure of a voltage-gated sodium channel came from bacteria, Arcobacter butzleri. Its ‘closed’ conformation, with four activated voltage sensors, was determined to 2.7 Å resolution and published in Nature in 2011.
How does the channel open and close?
In 2012 the structure of an ‘open’ bacterial form of the sodium channel was solved providing insight into how the channel opens and closes.
More information:
Learn how crystallography has improved our everyday lives.
You can also see a crystal structure a day here and find out how you can see a life-size crystal structure Crystals in the City exhibition.
About the author: Dr Julia Archbold
Published: 22/07/2014