Imagine having a pair of sunglasses that can detect dangerous levels of CO2 that are invisible to the human eye? ANSTO's research into chemical sensors for CO2 might make this possible in the future.
 |
Introduction
Excessive exposure to high levels of carbon dioxide (CO2) can endanger human health and even be fatal. As CO2 is invisible to the naked eye it can only be monitored using electronic equipment. Therefore we are developing chemical sensors for CO2 based on a spiropyran molecule with an amidine moiety conjugated to its backbone, that have generated a single molecule that is highly sensitive to the presence of CO2 and more importantly can be used to visually detect toxic levels of CO2 in the air.
A spectrum of colours, from purple to yellow, can be obtained depending on the concentration of CO2 being introduced. Just like photochromic (light-responsive) molecules darken UV sensitive sunglasses when exposed to the sun, this research has the potential to develop products that will change colour when exposed to high levels of CO2.
Photochromic molecules
Photochromic dyes (e.g. spiropyran – Fig. 1. See Image Library below) are light-responsive molecules. They reversibly transform between two forms which have exactly the same atoms but different structures, also called photo-isomerisation.
The light effect results in clear and coloured states, where molecular structure and polarity are dramatically different. When irradiated by UV light, spiropyran molecules adopt an ionic, planar merocyanine (MC) form and a deep purple colour; while under heat or visible light they revert to the neutral, orthogonal spiro (SP) form and are colourless.
Commercially, photochromic compounds have been applied in ophthalmic lenses and consequently, substantial research has concentrated in this domain [1]. More recently, the remote triggering and reversible behaviour of these compounds has led to their evaluation in numerous applications such as the modulation of stem-cell attachment and self assembly among other biological and chemical processes.
Carbon-dioxide and nitrogen gases as new triggers for spiropyran systems
Carbon dioxide (CO2) has excellent potential to act as a new mild stimulus that can be easily added and removed from solutions of photochromic molecules.
We have found that the photochromic effect of spiropyran molecules can be reversibly activated/deactivated by the addition and removal of CO2 from solutions of spiropyran containing an amidine species (e.g, DBU) [2].
The spiropyran/amidine complexes are yellow in solution and non-photochromic, but become photochromic again when CO2 is subsequently added to the solution (Fig. 1). The CO2 is readily removed by inert gas sparging which leads to the deactivation of photochromism and the reappearance of the yellow colour.
Our work suggests a number of applications in developing bio-responsive materials based on CO2 gradients found in industry and nature.
Research currently taking place at ANSTO is looking at ways to develop chemical sensors that can be used to visually detect toxic levels of CO2 in the air.
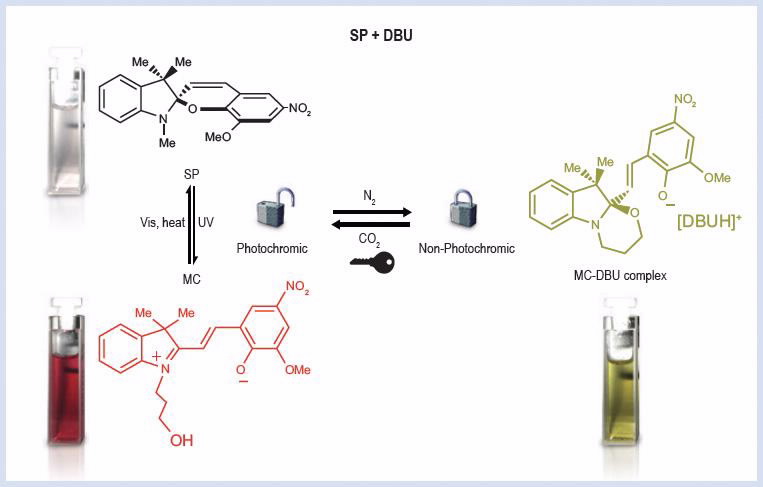 |
| Different forms of spiropyran (SP, MC and MC-DBU) and the reversibility of their associated reactions with different stimuli |
Photochromics and molecular logics
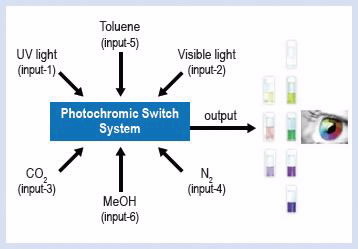
Other photochromic dye classes such as spirooxazines and chromenes are not affected by the CO2/amidine stimulus. As a result, we have developed a system of molecular switches based on mixtures of spirooxazine, chromene and spiropyrans that respond differently to UV and visible light as well as CO2 (Fig. 2).
This allows the photochromism of some molecules to be selectively preserved while reversibly deactivating the photochromism of others.
We have investigated the potential of our new solution systems for the application in molecular logics. Essentially, the ON or OFF states of solution photochromism represent “1” or “0” of a digital binary code. Different combinations of stimuli (inputs) give unique visual response (output), which is a blend of different individual colours (Table 1).
Traditionally, molecular logics suffer from dilution and fatigue effects which make the systems non-reversible.
In our system, the use of benign triggers such as visible light, UV, CO2 and N2 gives us a more robust logic system that does not suffer dilution effects, and hence becomes truly reversible [3].
 |
| Multiple inputs for the same solution that can display eight different output colours, all of the changes are fully reversible allowing a more robust molecular logic-system |
A new carbon dioxide sensor
CO2 is an environmental hazard in many areas and a simple visual method of detection remains elusive.
Monitoring the air quality continuously, where the output Figure 2 is monitored by the naked eye and no assay kit or instruments are required would be very beneficial in remote and confined places. We have developed a new visual probe for CO2 based on a spiropyran molecule with an amidine moiety conjugated to its backbone (SP-Am).
This has generated a single molecule that is highly sensitive to the presence of CO2 and more importantly can be used to visually detect toxic levels of CO2 in air; essentially acting as a modern-day molecular canary [4].
A spectrum of colours, from purple to yellow, can be obtained depending on the concentration of CO2 being introduced (Fig. 3). Also the solution is sufficiently sensitive for which colour changes are observed simply by having the CO2 in the air above the solutions.
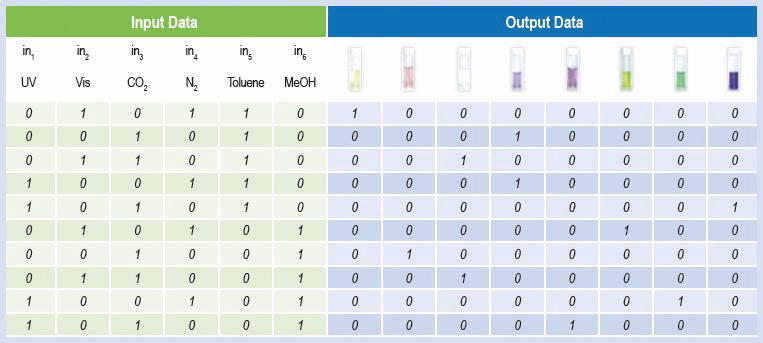 |
| Truth table for a mixture of chromene, spirooxazine and spiropyran with amidine, where a 0 indicates that the corresponding signal is off and a 1 that is on [3] |
Future work
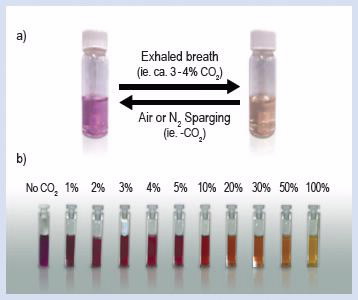 |
a) Co2 present in exhaled breath is enough to reversibly change the colour of dilute SP-Am solution, b) Gradual change in colour of SP-Am solution when increased concentration of CO2 is introduced [4] |
Development of new applications based on these photochromic switches is expected to be widespread.
This is in addition to finding roles in fundamental scientific studies. We have opened the door to a new method of triggering response of photochromic spiropyrans, as well as, providing a direct visual method for the detection of hazardous levels of CO2.
The path of absorption of the CO2 in solution for example can be visually monitored as it diffuses down an unstirred column of Sp-Am in solution. Such a system acts as a CO2 clock where the length of the yellow coloration provides an indication of time since CO2 became present above the solution.
Example of future work in this area would be focused on fundamental studies of CO2 diffusion in liquids; as well as applications of these dyes in a solid matrix form.
Authors
Tamim Darwish1, Richard Evans2, Michael James1 and Tracey Hanley1
1ANSTO, 2CSIRO Materials Science and Engineering
References
- Evans, R. A., Hanley, T. L., Skidmore, M. A., Davis, T. P., Such, G. K., Yee, L. H., et al. (2005). The generic enhancement of photochromic dye switching speeds in a rigid polymer matrix. Nature Materials, 4(3), 249-253.
- Darwish, T. A., Evans, R. A., James, M., Malic, N., Triani, G., & Hanley, T. L. (2010). CO2 Triggering and Controlling Orthogonally Multiresponsive Photochromic Systems. Journal of the American Chemical Society, 132(31), 10748–10755.
- Darwish, T. A., Evans, R. A., & Hanley, T. L., Spiropyran, chromene and spirooxazine, mélange á trois: Molecular logic systems through selective and reversible deactivation of photochromism mediated by CO2 gas. Dyes and Pigments (2011), Article in Press doi: 10.1016/j.dyepig.2011.03.021.
- Darwish, T. A., Evans, R. A., James, M., & Hanley, T. L. (2011). Spiropyran-Amidine: A Molecular Canary for Visual Detection of Carbon Dioxide Gas. Chemistry-A European Journal, Article in press doi: 10.1002/chem.201101723. (Published on online 29 Aug 2011)


