 |
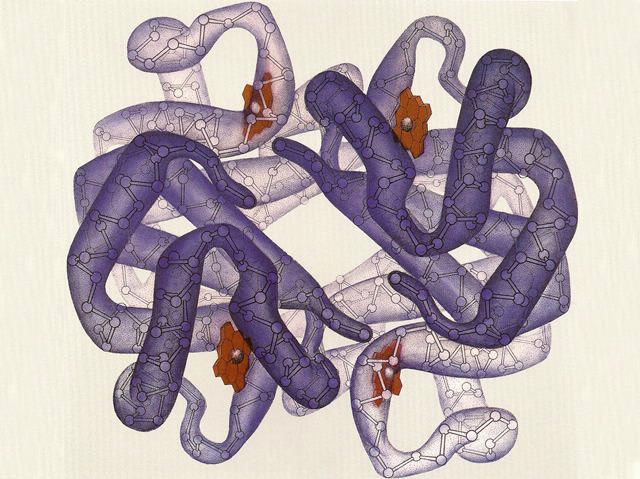
| A representation of a haemoglobin protein, showing the four haem groups (red), each surrounded by a polypeptide subunit. These subunits partly unfold to allow efficient oxygeb binding and release. Image credit: Irving Geis. |
An international research team including physical bioscientist Dr Chris Garvey from the Bragg Institute located at the Australian Nuclear Science and Technology Organisation (ANSTO) in Lucas Heights used neutron scattering techniques to discover the key mechanism explaining thermal fluctuations of haemoglobin in a number of species, including Australia's native Duck-billed platypus.
Their research was published in the journal J. R. Soc. Interface.
Haemoglobin is the iron-containing pigment of the red blood cells. Its function is to carry the oxygen from your lungs to your tissues.
Blood samples taken from a human, a duck-billed platypus, a chicken and a salt-water crocodile reveal haemoglobin molecules evolved to carry oxygen best at different body temperatures in different species.
The work conducted by researchers from Institut Laue-Langevin (ILL), Aachen University of Applied Sciences (FH Aachen), the Centre National de la Recherche Scientifique (CNRS) in Paris and FRMII in Germany and led by Dr Chris Garvey from the Australian Nuclear Science and Technology Organisation (ANSTO) involved the use of neutron scattering techniques to study blood samples at the atomic level.
Oxygen is taken up by, and released from, iron atoms in the haemoglobin and this is understood to happen most efficiently when the haemoglobin molecule partly unfolds and softens.
Dr Garvey and colleagues studied the way haemoglobin makes this transformation in different species with different body temperatures.
“We’ve been looking at how its softness changes as a function of temperature,” Dr Garvey said.
To obtain the best profile of how this may work in different species with different body temperatures, the flexibility and stiffness of haemoglobins was studied in three endotherms with different body temperatures (platypus, domestic chicken, and human) and an ectotherm (salt-water crocodile) and measured by incoherent elastic neutron scattering at Institut Laue-Langevin (ILL) in Paris and FRMII in Germany, while the amino-acid sequences that determined these properties were interpreted by complementary coarse-grained computer simulations.
What was revealed is that changes in the conformational dynamics of the molecule occurred at the exact body temperature of each endotherm.
This meant that, at the body temperature level, the protein becomes more flexible, allowing an easier pathway for gas molecules such as oxygen to diffuse in and out of the haemoglobin structure.
In the case of the salt-water crocodile, little change in haemoglobin flexibility with temperature was observed.
The findings, which explain how evolution has optimised the vital job haemoglobin carries out, within different species, could prove particularly interesting to biological, bioengineering and biomedical research on red blood cells.
Check out Science Daily, ABC Science and Innovation Digest for more reports on this research.
Published: 03/08/2012


