Ever wondered why graphite and diamonds are different when both are made from carbon?
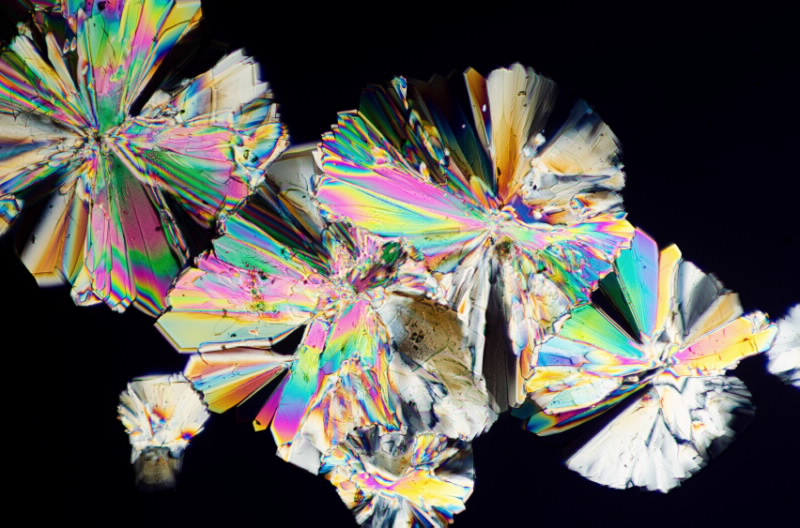 |
| Crystals have been used to improve everything from batteries to computers |
This story was originally published in the daily telegraph
Discover the answer – and learn more about the science behind it – at events planned across Sydney this month as scientists celebrate 2014 as the International Year of Crystallography.
“Most people know everything is made of atoms, but these atoms don’t flow freely in a material; they’re ordered,” says Dr Neeraj Sharma from the University of NSW School of Chemistry.
“In the case of graphite and diamonds, it’s the order of these atoms that determines their properties – that determines why one is black and brittle and the other is hard is shiny.”
Crystallographers seek to understand these structures and to replicate them in other materials – to make lithium batteries last longer, for example, or aircraft materials stronger.
“And everything we know about computers is due to crystallography,” Dr Sharma says.
As part of the celebrations the crystals in the city exhibition is launching from 18 August, during national science week and features giant crystal sculptures like molybdenum, diamond and perovskite. You can see the crystal sculptures at various locations around Sydney like Australian Museum, Royal Botanic Gardens and Centennial Parklands.
“I hope we can give people an appreciation of all the weird and wonderful things you can do with crystallography,” Dr Sharma says. “Science is about explaining nature in the simplest way possible – and then using that knowledge to build something much better.”
Learn more about crystal structures.


