The study of radiation damage is not only essential for the development of new materials used in reactor design, but also for designing new waste forms. In the following, we highlight our study of titanium dioxide, a model system with different polymorphs at room temperature. This allows the change in structure with no change in composition, to be studied and better understood, and then used to understand the effects on structure on radiation.
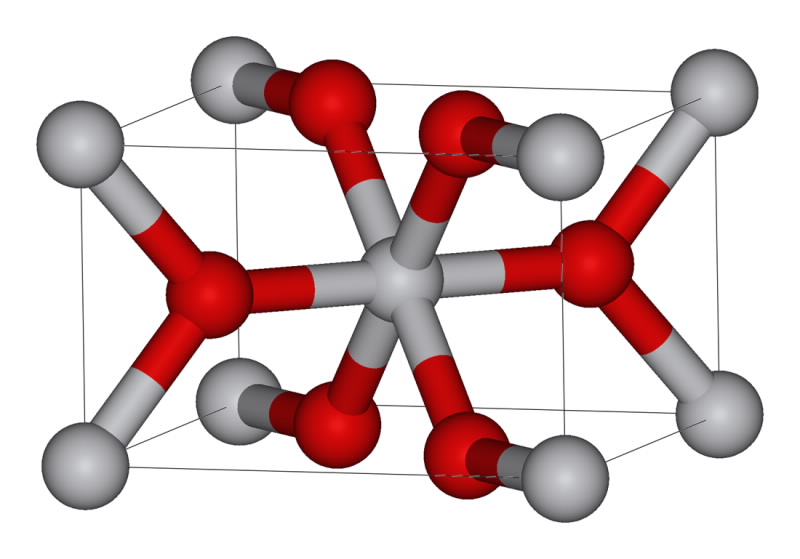 |
| Titanium oxide structure. Credit: Wikipedia |
Why we study radiation damage
The effects of radiation damage on materials are vital factors in both designing new waste forms, and new materials for use in future reactor designs, e.g. GenIV (Generation IV reactor designs incorporate advanced materials for cladding and structural components) and fusion.
For any material to be used in future design, its ability to withstand high levels of radiation must be understood, and if possible predictable. As part of this process we study damage in materials using both experimental and molecular-dynamics simulation techniques, enhancing predictive capabilities.
As models they can be applied to future waste forms and reactor materials, thus increasing the usability of a material over long periods of time.
Three Polymorphs of TiO2
In this study we focused on anatase, rutile and brookite, the three commonly found structures of TiO2. Polymorphs are formed under different conditions and have different structure, but they do not change chemically. Rutile is more stable than anatase, whereas brookite is difficult to assess as it is formed under high pressure and temperature in nature. Due to its simplicity, TiO2 is a model compound that allows the change in structure, without a change in composition to be investigated, and linked with recovery from radiation damage.
The samples were irradiated, using the in situ ion irradiation facility at the Argonne National Laboratory (IVEM-TANDEM), at different temperatures providing a means by which the recovery from damage can be correlated with temperature and the kinetics for recovery determined, see Figure 1. The results can then be used to calculate the activation energy for recovery that can be used in predictive studies.
When any material is irradiated the fluence required to amorphise the material is defined as the critical fluence (Fc). When irradiated at increasing temperatures, a temperature is reached whereby it is not possible to amorphise the material, i.e. the recovery process is quicker than the damaging process. This is called the critical temperature (Tc) and is obtained by analysis of the critical fluences at different temperatures. The lower the critical temperature the more rapid the recrystallisation is and thus the more stable to damage, a high Tc indicates a material which is easily amorphisable, and therefore unlikely to be used in reactors.
Our findings after irradiation
The studies showed that each of the structures behaves differently, for example synthetic rutile at 50 K was impossible to amorphise (indicating a Tc lower than 50 K), while a natural rutile (with impurities) was amorphisable with difficulty, whereas anatase and brookite were significantly easier. The structures of rutile, anatase and brookite are based on TiO6 octahedra, in different arrangements, e.g. rutile has 2-shared edges between octahedra, while brookite and anatase have 3 and 4.
The octahedra themselves have differing degrees of asymmetry with rutile being the most symmetric and anatase the least. The damage/recovery at 50 K can be directly contributed to the TiO6 octahedral connectivity, octahedral distortion, and the volume expansion from crystalline to amorphous. The tolerance was found to change anatase < brookite <rutile with the decrease in octahedral-edge sharing, and a subsequent increase in volume expansion between crystalline and amorphous forms. Applying molecular-dynamics based simulations a similar trend was found agreeing with the experimental results [1], shown in Figure 2 for anatase and rutile.
Molecular-dynamics simulations took an amorphous region within a crystalline phase, and raised the temperature to recrystallise the structure back. After the samples were irradiated and analysed (shown in Figure 3), there was a difference from the results obtained at 50 K. At 50 K synthetic rutile was found to be the most tolerant, while anatase was the least tolerant, with brookite in between.
Using Tc as a measure for damage resistance brookite was most tolerant (discounting the previously unamorphisable synthetic rutile), and anatase the least, with rutile inbetween [2]. These differences are complex, but can be linked to the changes in general, rutile is more stable than anatase when the structure is considered, while brookite is difficult to assess as it is formed under high pressure and temperature in nature.
Rutile, which is more resistant to damage than anatase, has the largest crystalline to amorphous volume change, and the most symmetrical TiO6 octahedral co-ordination, while anatase has the smallest volume change and the most asymmetric TiO6 octahedral co-ordination.
Such structural information can now be added to predictive models being developed for materials in high radiation fields, and shows that structure directly impacts on the tolerance of materials.
Outlook
The results from this work are being incorporated into models describing how materials recover from radiation damage. The models are being used in predicting the effects of radiation on Y-Ti-O oxides, which are used as additives in ODS materials (Oxide Dispersion Strengthened materials have oxides added to help improve the performance), which have been proposed for use in future reactor technologies.
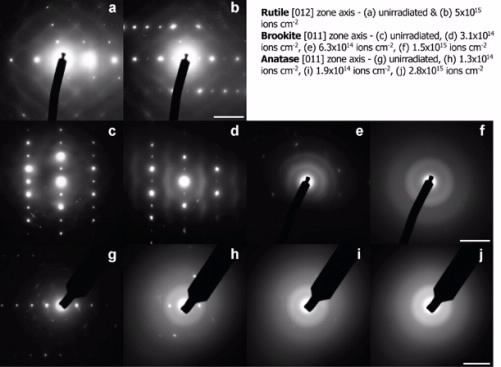 |
| Figure 1: Effects of increasing radiation damage on samples observed using Transmission Electron Microscopy (TEM). The numbers refer to how many ions have been impacted upon the sample within the microscope. |
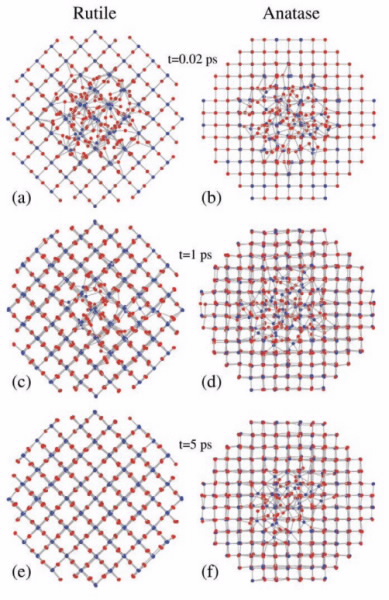 |
| Figure 3: The results from the molecular- dynamics simulation of rutile and anatase (brookite has been omitted for clarity). The results show that rutile (a), (c), and (e), the most symmetric, recovers more rapidly than anatase (b), (d) and (f) the most asymmetric. |
Karl Whittle, Mark Blackford, Zhaoming Zhang, Gordon Thorogood, Jeff Ferenczy, Meng Jun Qin, Eugenia Kuo, Robert Aughterson and Joel Davis
ANSTO
References
- Lumpkin G. R.; Smith K. L.; Blackford M. G.; Thomas B. S.; Whittle K. R; Marks N. A.; Zaluzec N. J., Physical Review B, 77, (2008), 212401.
- Lumpkin G. R.; Blackford M. G.; Smith K. L.; Whittle K. R.; Zaluzec N. J., Ryan E. A.; Baldo P., American Mineralogist, 95, (2010), 192-195.
Published: 06/02/2009


