Research being conducted at ANSTO is helping to refine the radioisotopes that will be used to target tumours in the next generation radiopharmaceuticals for treatment and diagnosis of cancer.
State-of-the-art radiopharmaceutical development requires radioisotopes of optimal specific radioactivity to maximize radiopharmaceutical uptake in the body.
Specific radioactivity is the ratio of radioactive atoms to the sum of non-radioactive and radioactive atoms of the chemical element. Optimising specific radioactivity allows more radioactive atoms to be delivered to the tumour. This enhances the image quality in patient scanning and improves effectiveness of endo-radiotherapy (tumour cell killing using internally deposited radiation) treatments.
In general terms, high specific radioactivity is important because it increases the fraction of radioactive atoms bound to the radiopharmaceutical precursor. This increases labelling efficiency, and reduces the amount of precursor necessary to maintain efficiency.
Additional precursor could have deleterious side effects when injected into patients.
High specific radioactivity 177Lu is a prerequisite to formulate radiopharmaceuticals targeting tumours for several different cancer treatments (neuroendocrine tumours, breast and colon cancer, and lymphomas).
Radioisotopes can be produced from a range of sources; cyclotrons, radionuclide generators and nuclear reactors. The advantages of nuclear reactors are large production capacity, easy target preparation and irradiation and robust operation.
The current expansion of targeted endo-radiotherapy is dependent on the availability of high specific radioactivity radioisotopes (such as 188W/188Re, 90Y and 177Lu) which can be produced in nuclear research reactors.
The yield (activity produced for each target irradiation or generator elution) of radioisotope production from cyclotrons, radionuclide generators and nuclear reactors is usually the only parameter used to justify using a particular nuclear reaction [1].
While the conventional yield evaluation provides high quantities of activity, it is insufficient to set up the optimal conditions for producing radionuclide products of the desired radiochemical quality. It does not take account of undesirable effects, e.g. target impurities and unwanted radioactive transformations.
Alternatively, we can assess the specific radioactivity by dealing with the relationship between the affecting factors and the inherent properties of the target.
Assessing this relationship we can optimise the irradiation for production of the highest specific radioactivity radioisotopes for various applications.
This is especially true for receptor/antigen targeted radiopharmaceutical preparation.
In addition, this method plays a complementary or even substantial role in the quality management and subsequent certification regarding the radioisotope product. For example, based on our theoretical specific radioactivity assessment results, we could calculate optimal conditions to produce 177Lu suitable for radiopharmaceutical preparations for targeted endoradiotherapy.
Better technology performance of radioisotope production, example 177Lu
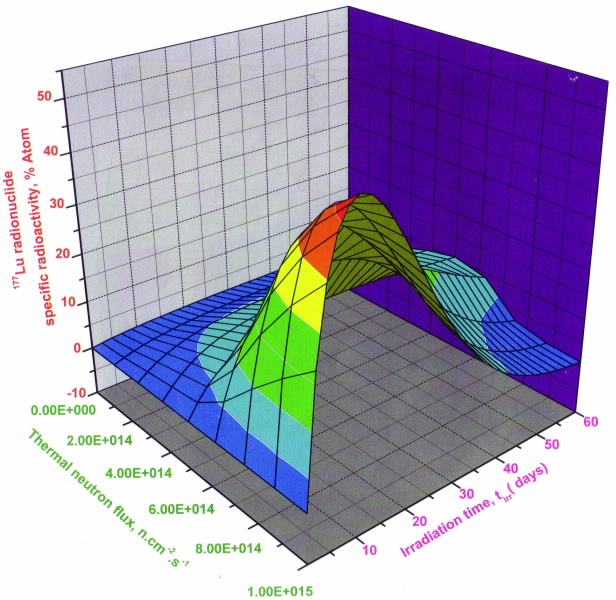 |
| Fig 1. Specific radioactivity of 177Lu produced from 176Lu targets as function of the irradiation time and thermal neutron flux, Target composition: 74.1%176Lu and 25.9 % 175Lu. |
The specific activity assessment of radioisotopes produced in a neutron–activated target is a complex issue and has not yet been fully solved. The targets used in the production of short-lived medical radioisotopes usually have high neutron-capture cross-sections.
Neutrons are readily absorbed by the target atoms and facilitate the conversion to the radionuclide.
These high cross section will provide the highest possible SA values. This fact causes a high “real” burn-up (conversion) of the target element. In particular, the short half-life of the beta-emitting radioisotope produced in the target hastens the transformation of the target nuclide to the produced radioisotope which in turn strongly affects the SA.
Neutron-capture characteristics, target impurities, side nuclear reactions, target burn-up and post-irradiation processing/cooling time are the main parameters affecting the SA of the radioisotope product. As a result of our recent study, these parameters have been incorporated into the format of mathematical equations for the reaction yield and SA assessment [2].
In this study, SA assessment was performed on the production of 177Lu from 176Lu and 176Yb enriched targets.
The irradiation conditions required for achieving a maximum yield and maximum SA value and the effect of several factors (such as elemental Lu and 174Yb/175Lu isotopic impurities) on the reduction of 177Lu SA were evaluated.
177Lu is a radioisotope of choice for endo-radiotherapy because of its favourable decay characteristics: a medium-energy beta decay of 497 keV with moderate range in tissue and half-life of 6.71 days. It also emits gamma rays of 113 keV and 208 keV which make it useful for imaging in vivo localisation with a gamma camera.
177Lu can be produced by two different routes, a direct route with the 176Lu (n, g) 177Lu reaction and an indirect route via the 176Yb (n, g) 177Yb (b- decay)→ 177Lu nuclear reaction-transformation. The direct route could be successfully performed in high neutron-flux nuclear reactors but these are available in only a handful of countries in the world.
Additionally, large burn-up of the target nuclide during high neutron-flux irradiation may cause a decrease in the specific radioactivity of the produced nuclide if the target contains isotopic impurities. The indirect route is used for production of “no carrier-added” 177Lu of higher specific radioactivity. In this case, the same reduction in specific radioactivity can occur if the target contains isotopic and/or elemental Lu impurities.
While being the best theoretical way to produce no carrier-added 177Lu with this reaction, we always obtain a 177Lu product of much lower specific radioactivity due to the use of an isotopically/elementally impure target.
Based on the theoretical SA assessment results obtained in our report, the optimal conditions for 177Lu production were set up to maximize the production of high specific radioactivity 177Lu product [2].
 |
Fig 2. Specific radioactivity of 177Lu produced from 176Yb target as function of the irradiation time and thermal neutron flux; target composition: 97.6% 176Yb, 1.93% 174Yb and 50 parts per million Lu impurities. |
Theoretical approach and methods of SA assessment [2]
Reactor-based radioisotope production usually involves two main nuclear reactions. The first one is the thermal neutron-capture (n, g) reaction.
This reaction (direct, see above example) does not lead to a radioisotope of another chemical element, but following radioactive b- decay of this isotope during target activation results in a decrease in both the reaction yield and atom numbers of the target chemical element.
The second reaction (indirect, see above example) is thermal neutron-capture followed by radioactive transformation S (n, g) Rx (b-decay) Ri (S is nonradioactive target nuclide; (n, g) neutron-capture nuclear reaction; Rx short-lived intermediate radioactive nuclide; Ri product radioisotope).
This reaction leads to a no carrier-added radioisotope (Ri) of another chemical element than the target chemical element (S).
The specific radioactivity assessment in the radioisotope production using the first reaction (with a simple target system) is simple. Careful targetry could avoid the side reaction S (n, g) Ry (b- decay) R which could result in the isotopic impurities R for the radioisotope Ri intended to be produced using the first reaction.
In this case, the specific radioactivity assessment can be simplified by investigation of the decline in specific radioactivity due to target nuclide burn-up, chemical element depression due to radioactive decay and isotopic impurities present in the target.
On the other hand the SA assessment in the radioisotope production using the second reaction (with complex target system) is more complicated.
The complexity of this targetry requires an analysis of the combined reaction system.
We can assess the effect of side nuclear reactions in this target system in addition to the three above mentioned factors that are involved in the simple target system.
In this case, the specific radioactivity assessment is best resolved by a comprehensive method of specific radioactivity calculation.
The complex target system is considered as a mixture of several radioactive sources of variable specific radioactivity; details on the method of specific radioactivity assessment for this mixture is formulated in reference [2].
Figures show the results of our calculations to determine the specific radioactivity of 177Lu radioisotope produced by the direct (fig. 1) and indirect (fig. 2) route as a function of the elemental and isotopic impurities of the 176Yb enriched target, the irradiation time tirr and neutron flux [2].
A defined maximum specific radioactivity value for each target and its irradiation condition and neutron flux was found for both production routes suggesting that the irradiation time could be optimised to obtain the highest specific radioactivity of 177Lu. The maximum specific radioactivity value was affected by a combined effect of 174Yb and elemental Lu impurities.
However, these results are only valid for a specific target composition and its neutron-irradiation conditions.
This theoretical assessment of specific radioactivity maximization is critical before starting the neutronactivation process. The optimised process would avoid lowering the specific radioactivity of 177Lu product by over-bombardment, and also avoid wasting expensive reactor-operation time.
Lastly, the post-irradiation processing time should be minimised to keep the specific radioactivity as high as possible. Fig. 3 shows an automated system for production of high specific radioactivity 177Lu.
Besides enhancing the speed of purification, this system has the added benefit of reducing operator absorbed dose. The details of this process have been presented in a number of previous publications [3-5].
|
| Fig 3. Equipment for production of 177 Lu solution of high SA from 176+175 Yb target bombarded with the OPAL reactor neutron flux. |
Author
Van So Le, ANSTO
References
- Rubinson, W. (1949). The Equations of Radioactive Transformation in a Neutron Flux. Journal of Chemical Physics, 17(6), 542.
- Le, V. S. (2011). Specific radioactivity of neutron induced radioisotopes: assessment methods and application for medically useful 177Lu production as a case. Molecules, 16(1), 818-846.
- Le, V. S. (2008). Alternative chromatographic processes for no-carrier added 177Lu radioisotope separation. Part I. Multi-column chromatographic process for clinically applicable. Journal of Radioanalytical and Nuclear Chemistry, 277(3), 663-673.
- Le, V. S., Morcos, N., Zaw, M., Pellegrini, P., Greguric, I., & Nevissi, A. (2008). Alternative chromatographic processes for no-carrier added 177Lu radioisotope separation. Part II. The conventional column chromatographic separation combined with HPLC for high purity. Journal of Radioanalytical and Nuclear Chemistry, 277(3), 675-683.
- Le, V. S., & Morcos, M. (2008). New SPE column packing material: Retention assessment method and its application for the radionuclide chromatographic separation. Journal of Radioanalytical and Nuclear Chemistry, 277(3), 651-661.



