
An ANSTO Planetary Materials scientist has used the Australian Synchrotron to identify the structure of a new material that could be crucial in understanding the hydrological cycle on Titan, the largest moon of Saturn, and important in assessing its potential habitability.
Helen Maynard-Casely used the Powder Diffraction instrument at the Synchrotron to determine the atomic structure of the new material, a co-crystal between benzene and ethane that forms at 90 degrees Kelvin (-179.2 °C), the frigid surface temperature on Titan.
The experiment was done in situ under cryogenic conditions similar to Titan.
The paper has just been published online in the International Union of Crystallography’s open access journal, IuCrJ.
The material, which is formed by molecules of benzene and ethane, had been first identified by her collaborators at the Jet Propulsion Laboratory in the US using another technique, spectroscopy.
“They had no way of knowing exactly what they had until the crystalline structure could be found,” said Maynard-Casely.
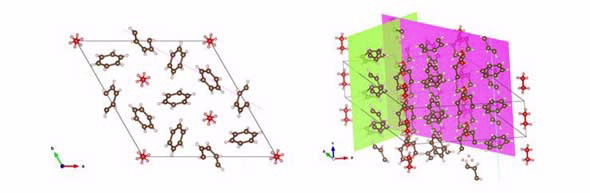 |
| The packing of molecules and planes of C–H…π interactions across the benzene:ethane co-crystal. In the representation of the benzene:ethane co-crystal, the ethane molecules are coloured red.The benzene:ethane and benzene structures have the planes of H…π interactions highlighted. |
Only the diffraction of X-rays, which interact with the electrons of this hydrogenous material, could provide information about the arrangement of atoms in a crystal lattice.
The structure of the new material is based on the structure of pure benzene; with its framework stabilised by attraction between hydrogen atoms at the edges of the molecule and the π-bonds in the middle,” said Maynard-Casely.
“These sorts of bonds haven’t been found in planetary materials before. And the surprise is that this bond is strong and may be as strong as when hydrogen bonds to oxygen (like in ice) at Earth temperatures,” she said.
In the new material, the benzene molecules form a framework that surrounds ethane molecules in a highly symmetric geometry. The benzene molecules actually form a framework with a channel, and the ethane molecules occupy this channel.
“The only reason the ethane would have done that is that there was some energy benefit.“
“It’s a marvellous concept that this host-guest structure is the lowest energy configuration, rather than benzene and ethane crystallising separately. It begins to make you think what other materials are possible,” said Maynard-Casely.
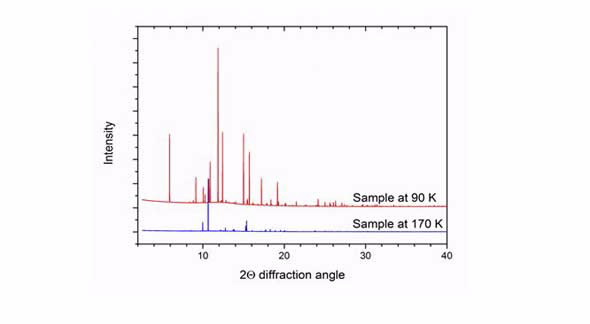 |
| Comparison of the diffraction pattern of the sample at 90 and 170 K. The pattern at 170 K corresponds to pure benzene, while the pattern at 90 K contains peaks from both benzene and the benzene:ethane co-crystal. |
“I had to stop thinking like an Earth geologist and start thinking like a Titan geologist, because at 90 K the physical and chemical processes that form materials is very different,” said Maynard-Casely.
The latest research about Titan suggests there is very little water-ice on the surface, although the thought is that a subsurface ocean of water exists between the crust and deeper layers of high pressure ice.
However, Titan does have a thick atmosphere - with clouds, lakes and seas, all thought to be made up of a mixture of methane and ethane, and mountains and dunes composed of grains of small hydrocarbons.
“The spacecraft Cassini discovered that Titan does have a hydrological cycle, but it is driven by methane and ethane rather than water like here on Earth,” explained Maynard-Casely.
“But, despite the fact the chemistry is very different, we see lots of similar features between Earth and Titan – one such feature is the dried-up lake beds on Titan,” said Maynard-Casely.
When lakes dry up on Earth, you are left with evaporite mineral, mixtures of salt and hydrates. If the liquid methane and ethane lakes evaporated on Titan, what would be left could be the co-crystal characterised by Maynard-Casely and the team.
The Titan evaporite has been the missing part of the geochemical cycle. “You have clouds and lakes but what is the intermediary?” asked Maynard-Casely.
“The ongoing challenge is to work out the materials on the surface of the planet in order to start explaining the surface features we see.”
The JPL team was also very interested to find out if the evaporite material might be the source of a dynamic transient feature in one of Titan’s hydrocarbon lakes, known as ‘magic island’ detected by radar. “It didn’t have the right density, unfortunately, to account for the appearance and disappearance of this feature.”
“We used to think the water-based hydrological cycle on Earth was unique, but we now have evidence of cycles on other bodies, like Pluto, most recently.”
In previous work, co-author Tuan Hoang Vu of the Jet Propulsion Laboratory worked out the kinetics of the surface temperature and the lakes on Titan.
“Processes take place at these temperatures but they are a lot slower,” said Maynard-Casely.
The research on the co-crystal will continue later this year when Maynard-Casely and colleagues from the Jet Propulsion Laboratory will deuterate the co-crystal and use neutron powder diffraction for further analysis.
http://dx.doi.org/10.1107/S2052252516002815
ANSTO offers neutron beam, synchrotron beam and materials facilities to support research in planetary science.
Published: 30/03/2016

