Neutron diffraction has graced the front cover of the prestigious chemistry journal Angewandte Chemie.
A team of researchers from the University of Zaragoza, the Institut Laue-Langevin, Grenoble, and the Bragg Institute has shown, for the first time, transport via the 200-year old "Grotthus Mechanism" of hydrogen atoms along a proton chain in a non-porous solid.
Movement of water and protons, including transport in confined spaces, is of considerable importance in a number of key areas of science and technology.
Water transport can be observed in diverse media from solids such as zeolites, through spongy clays, to living cells, and in various contexts such as molecular-storage materials, catalysis, and soil science.
Proton migration, which is often related to water transport, is of current interest in energy applications, while water transport in biology is of special importance, especially in cell membranes in which sophisticated structures permit the passage of water while blocking ion transport.
A common form of proton migration is the diffusion of water into absorbing materials, either via channels and cavities or via some sort of pathway which involves a proton cascade.
Already 200 years ago, Grotthuss, in trying to explain electrolysis in water solutions, proposed concepts that with time took the form of a hopping-reorientation mechanism in which the movement of ionic H+ is supposed to occur involving concerted proton “hand-off” from one water molecule to the next in a chain.
Such a mechanism has been invoked to explain proton mobility in a number of different processes, very often in solution, but has not always been supported by experimental evidence.
The full reference for the on-line paper is: “Proton Cascade in a Molecular Solid: H/D Exchange on Mobile and Immobile Water”, S.C. Capelli, L.R. Falvello, E. Forcén-Vázquez, G.J. McIntyre, F. Palacio, S. Sanz, M. Tomás, (2013), Angew. Chem. Int. Ed. DOI: 10.1002/anie.201309817.
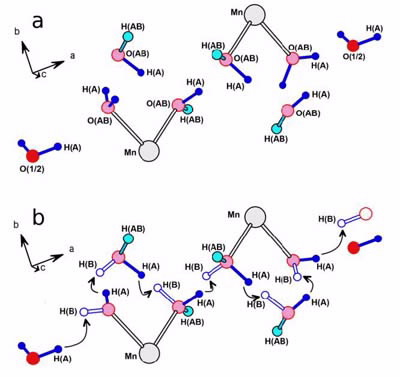 |
| Schematization of the Grotthuss hopping mechanism for the hydrogen atoms in the structure of [Mn(H2O)6]2n{[Mn(H2O)5]Mn4(citr)4[μ-Mn(H2O)4]}n•8nH2O. a) starting structure: the hydrogen atoms represented in dark blue are those subjected to the hopping mechanism; b) the arrows indicate the path of the hydrogen atoms in the structure and the open circles the new position of the hydrogen atoms. |
Published: 21/01/2014


