Humans are placing ever greater demands on metals to produce stronger and more durable products but how extreme can their performance testing? Researchers from ANSTO and the University of Wollongong put some metals to the test.
Introduction
The performance of metals under extreme operating conditions, such as high temperature, pressure, shock and mechanical load, is strongly linked to their microscopic structure.
Modern research aims to make materials withstand even tougher conditions.
While conventional metallurgical analysis uses quench-arrest (rapid cooling) techniques to study microstructural evolution, neutron and synchrotron X-ray facilities allow the study of those as well as the response of a material to mechanical deformation in situ and in real time. This tremendously advances the field, as many deformation mechanisms occurring at the atomic scale cannot be observed in other ways.
One example relates to changes that occur in a zirconium alloy at high temperatures.
Why research on metals
Since the Bronze Age humans have melted and processed metals to manufacture advanced products for the most diverse applications in our daily life, with demanding mechanical strength, ductility, durability and ease of use.
Examples of materials that have been developed to be used under these extreme conditions are high-strength steels to produce thinner, and therefore lighter automotive parts in addition to improved shock-absorbing capacity upon impact; high-strength, light-weight alloys for the aerospace industry to reduce fuel costs; high-temperature materials for more effective turbine performance; radiation-resistant materials for applications in the nuclear industry; materials possessing bio-compatibility in addition to complying with mechanical demands for medical implants; and micro-mechanical components for mechatronic devices.
Influence of microstructure on mechanical properties
Most metals comprise crystallite grains, which form the building blocks of a useful piece of material.
These crystallites can be arranged in a multitude of ways and the same or multiple phases can be moulded and shaped by a variety of mechanical deformation processes. The atomic mechanisms by which plastic deformation can occur are slip, twinning, vacancy diffusion, grain boundary sliding and stressinduced phase transformations.
Resistance to plastic deformation can be enhanced by invoking distortions into the crystal structure and creating barriers to lattice dislocation motion. Examples are solute elements, precipitates, grain boundaries, dislocation structures, and phase boundaries which must be circumvented in order to cause permanent deformation.
Microstructure evolution through heat treatment
Thermo-mechanical processing influences the microstructure of metals, and heat treatment at elevated temperature can, for example, create precipitates making the material stronger. On the other hand, heat treatment of already deformed material might lead to recovery, recrystallisation and grain growth, which softens the material – a problem often encountered upon welding and joining.
In order to assess the impact of deformation and heat treatment on the resulting mechanical properties of a material, one must fully understand the underpinning fundamental physical mechanisms such as dislocation movement, phase transformations that might occur, diffusional processes and the response of the material to local stress concentrations.
In situ neutron diffraction
In our present studies, we analyse the thermo-physical behaviour of metallic materials by the use of in situ neutron diffraction techniques. These techniques allow us to probe in real time the evolution and changes in microstructure, which are then correlated with complementary characterisation methods in order to better understand the fundamental driving forces for the material behaviour.
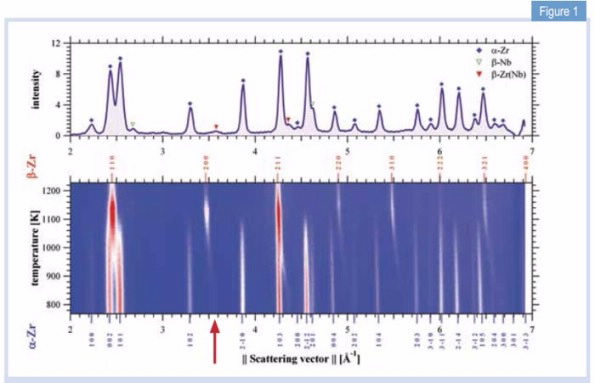 |
| Fig 1. Diffraction pattern of Zr-2.5Nb at room temperature (top) and its temperature evolution (bottom) with intensities coded in color levels. |
Most of the in situ data, recorded while the material was heated, held at temperature and cooled, was acquired using the high-intensity diffractometer Wombat [1] at the OPAL facility at ANSTO.
In addition high-resolution scans at preselected holding temperatures were obtained using the Echidna diffractometer [2].
Materials that have been studied by these techniques include zirconium alloys used in the nuclear industry; titanium alloys for use in bio-medical applications, as well as in high-strength, light-weight applications in the aerospace industry; titanium-aluminium intermetallics for use in high-temperature turbines in aerospace engines [3]; and high-strength steels designed to reduce mass and increase safety in the transportation industry [4].
A common theme in these investigations is the attempt to better understand how enhanced mechanical properties can be obtained by alloying, heat treatment and thermo-mechanical processing.
In addition, we have studied the way in which both diffusive and displacive phase-transformations play a role in the development of microstructrue.
Example: neutron diffraction of a zirconium alloy [5]
In thermodynamic equilibrium, the important nuclear reactor material, Zr-2.5Nb (mass %) consists of an α-Zr-rich phase with a hexagonal close-packed structure and a β-Nb-rich phase with a body-centered cubic structure at room temperature.
This two-phase material has been widely researched with respect to microstructural characterisation following a variety of, high-temperature deformation and variant selection during phase transformation.
However, the underlying crystalline properties, e.g. the lattice parameters and thermal expansion of the different phases during the phase transformations, have not been determined in situ and in real time. Using a vacuum furnace on Wombat, we recorded for the first time, the crystalline properties of Zr-2.5Nb during the α-β phase transformation.
Fig. 1 shows a stacked sequence of individual diffraction patterns with temperatures on the vertical axis, and intensity indicated by colour. The fraction of the β-Zr(Nb) phase starts to increase above the eutectoid temperature of Teu = 883 K, i.e. the lowest temperature and specific concentration, at which the system undergoes the transformation, while the α-Zr vanishes totally at Tβ = 1133 K; in accordance with the phase diagram.
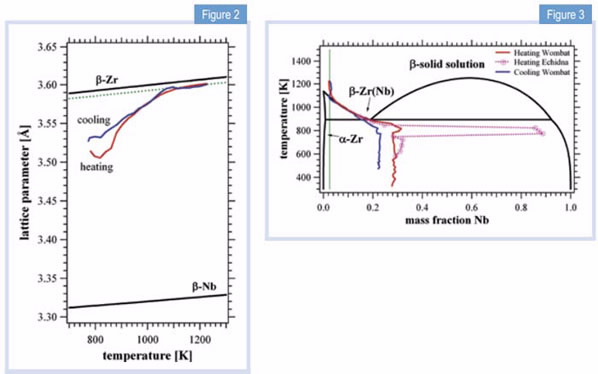 | |
| Figure 2: Lattice parameters for the pure element β-phases as a function of temperature, including the experimental data of the β-Zr(Nb) alloy upon heating and cooling. The dotted line would correspond to a homogeneous solid solution of Zr-2.5Nb. | Figure 3: Nb concentration of β-Zr(Nb) phase during heating and cooling with the rate of 2 K/min. The black line is the accepted Zr-Nb phase diagram; the red line represents the continuous heating process; the blue line represents the continuous cooling process while the dotted pink line is obtained by data acquired during 60 min temperature holding steps. |
Apart from the structures, the effect of the alloying element Nb on this phase transformation process has been investigated. The arrow in Fig. 1 locates the 200 peak of retained β-Zr(Nb).
During the phase transformation the β-Zr(Nb) peak shifts to the position of the expected β-solid solution peak.
The strong changes in lattice parameter testify to variations in the Nb concentration of the β-Zr(Nb) phase during the α−β transformation.
We can evaluate the change in lattice parameter by a combination of Vegard’s law (the linear variation of lattice parameter with concentration) and thermal expansion, Fig. 2, to obtain the Nb concentration in the β-Zr(Nb) phase, which is superimposed as a trace on the phase diagram in Fig. 3. Initially, the β-Zr(Nb) phase contains 28% Nb, but as atomic diffusion becomes more important during heating, Nb segregates up to 90% on 60 min holding steps. It then dilutes again to meet the eutectoid point at 18.5% and Teu, following from there on the β-transus line and eventually reaching the composition of the solid solution.
The path reverses upon cooling and the concentration is quenched, in this case at 23% for the applied cooling rate. Since composition influences the microstructure, such knowledge of the kinetics is extremely important for the establishment and prediction of heat treatment in a manufacturing or welding process.
Desired concentrations can be fine-tuned and the operating temperatures for a work piece can be defined in a more accurate way.
Conclusion
Neutron diffraction studies not only provide quantitative phase analysis, but are also a method of analysing the migration of alloying elements and their kinetics during phase transformations in situ and in real time.
Evolution of lattice-parameter changes elucidate the variation of atomic concentrations during the phase transformation, which are dramatic during the eutectoid reaction in the Zr-2.5Nb system.
Through analysis of the diffracted intensity and peak positions of different phases, a Materials engineering complementary image of the phase transformation process can be produced during heat treatment.
The significance of this study is to disclose the transient behaviour relevant to the phase transformations in a Zr-2.5%Nb alloy. Detailed knowledge of phase diagrams is vital for any materials studies.
Here, we have clearly recorded the decomposition behaviour in a fast, uncomplicated and concise way; this cannot be achieved by conventional studies which are usually obtained from ex-situ X-ray diffraction or dilatometric tests.
This approach benefits investigations of a multitude of multi-phase materials, and is not just limited to metals.
Authors
Klaus-Dieter Liss1, Kun Yan 1,2, Saurabh Kabra1,Lisa Thoennessen1,2, David G. Carr1, Robert P. harrison1, Rian Dippenaar2
References
- A.J. Studer, M.E. Hagen, T.J. Noakes, Physica B: Condensed Matter 2006, 385-386, 1013.
- K.-D. Liss, B. Hunter, M. Hagen, T. Noakes, S. Kennedy: “Echidna—the new high-resolution powder diffractometer being built at OPAL”, Physica B: condensed matters 385-386/part 2 (2006), p. 1001-1002. Doi/10.1016/j.physb.2006.05.322.
- Saurabh Kabra, Kun Yan, Svea Mayer, Thomas Schmoelzer, Mark Reid, Rian Dippenaar, Helmut Clemens, Klaus-Dieter Liss: “Phase transition and ordering behavior of ternary Ti–Al–Mo alloys using in situ neutron diffraction”, International Journal of Materials Research 102/6 (2011), p. 697- 702, doi/10.3139/146.110528.
- K. Yan, D.G. Carr, M.D. Callaghan, K-D. Liss, H. Li: “Deformation mechanisms of twinning induced plasticity steels: In situ synchrotron characterization and modeling”, Scripta Materialia 62/5 (2010), p. 246-249. doi/10.1016/j.scriptamat.2009.11.008.
- Kun Yan, David G. Carr, Saurabh Kabra, Mark Reid, Andrew Studer, Robert P. Harrison, Rian Dippenaar, Klaus-Dieter Liss: “In situ Characterization of Lattice Structure Evolution during Phase Transformation of Zr-2.5Nb”, Advanced Engineering Materials, 13/9 (2011), p. 882-886 (+ Back Cover Page). doi/10.1002/adem.201000350 + doi/10.1002/adem.201190023.
Published: 06/12/2012


