You are about to enter the strange world of coordination geometries, where energy and atomic bonding interplay to give materials unusual properties, such as negative thermal expansion (you heat something and it gets smaller) and the ability to capture and store gas molecules in nano-sized pore spaces.
Principal research and neutron-scattering instrument scientist Vanessa Peterson has collaborated on a paper just published in Nature Chemistry that reports the discovery of a new torsion spring-like mechanism in a series of coordination frameworks that has implications for the strategic design of future materials with exceptional mechanical properties.
The series of materials has the largest linear compressibilities of any crystalline compound, and the volume compressibilities are exceeded only by caesium, rubidium, and xenon. These compressibilities are both exceptionally large and sustained over a broad pressure range spanning at least 1 GPa.
The work is the product of a long-standing collaboration with Cameron Kepert at the Molecular Materials Group at the University of Sydney, with the first author Samuel Duyker co-supervised by Kepert and Peterson during his PhD before taking up a postdoctoral position with Peterson, when this work was performed.
“Understanding these multifunctional materials with the aim of controlling their range of useful properties is of great interest to the energy industry, but the research brings fundamental knowledge of the atomic and molecular mechanisms, which has broader application” said Peterson.
In contrast to the shortening of bonds that occurs in conventional materials, compression in the new series of materials is achieved through structural deformation. The unusual new mechanism was found in a series ‘coordination frameworks’—named for the coordinative bonds linking organic molecules and metal atoms. The torsional mechanism is unlike anything seen in any other material, being fundamentally distinct from other compressible frameworks.
The team first became interested in the lanthanoid frameworks because of their unusual coordination geometries.
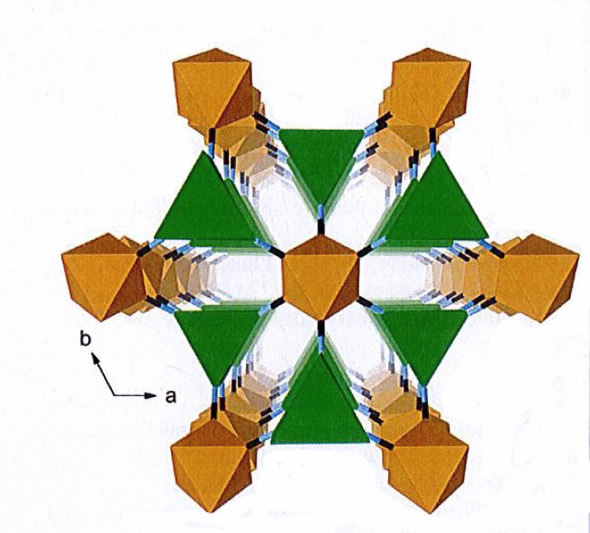 |
| The hexagonal structure of LnFe (CN)6 consisting of alternating LnN6 trigonal prism (green) and FeC6 octahedra (orange). |
“The material is a porous framework, constructed from two types of metals that are connected by cyanide bridging ligands. The coordination environment of the lanthanoid is inherently very unstable, but exists because it is stabilised by the overall framework connectivity.”
Torsional compression mechanism revealed
“Near 1 GPa, a change in lattice geometry occurs in the lanthanoid framework through a complex mechanism involving dramatic structural distortion,” said Peterson.
The LnN6 units acting like torsion springs are synchronised by rigid Fe(CN)6 units acting like gears. The LnN6 twists away from its original trigonal prismatic geometry becoming octahedral. These LnN6 units act as torsional centres that coil dramatically under pressure and enable extreme compressibility in combination with chemical and thermal stability for the first time.
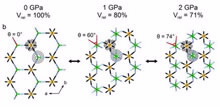 |
| A projection showing how the torsion spring-lke YN6 units twist from trigonal prismatic geometry at ambient pressure, becoming octahedral under pressure, and then towards trigonal prismatic again at higher pressures. |
“There is competition between two relatively strong effects. A balance is achieved between the significant stored energy in the locally unstable lanthanoid coordination and the opposing force of the hexacyanidoferrate units, which strongly prefer not to change.”
“These lanthanoid frameworks compress by about 20% in volume at the relatively low pressure of 1 GPa, one of the largest known pressure responses for any crystalline material,” said Peterson.
This represents a new paradigm in the design of materials with anomalous mechanical behaviours.
“There are a range of properties that can be tailored through choice of construction units. Selecting from a range of metal nodes and ligands gives control over the material’s porosity, topology, that is, the way that the pores are connected, the chemical functionality, and flexibility of these units.”
“Because the contribution of each part or unit of the framework to these material properties are known, they can be controlled, and tailored to particular functions. With this knowledge, you can gain insight into how to structurally engineer the properties you want at the molecular level,” said Peterson.
Of great importance in understanding the details of the compression mechanism in this material series was the validation of computational models using neutron scattering. The combination of the two in this approach is well-known and very powerful.
The neutron powder diffraction portion of the research was done on ANSTO’s high intensity powder diffractometer, WOMBAT, for which Peterson is an instrument scientist.
“The unique properties of neutrons allowed the neutron scattering experiment to be performed on a bulk sample, which was very useful, and provided great contrast in the scattering power of the elements,” said Peterson.
Temperature studies reveal something unusual in coordination geometry
In earlier work, the team had found that the co-efficient of thermal expansion correlated linearly with the ionic radius of the lanthanoid in this family of materials. As part of the study, they substituted a range of elements, including yttrium, holmium, and lutetium, into the coordination site changing the coordinative bond strength.
“We measured the overall property of thermal contraction or expansion using neutron scattering and computational methods to understand the mechanism. We found that there are low energy vibrational modes that give rise to this negative thermal expansion property. One of those modes was extremely unusual,” said Peterson.
“In theory you can tune the thermal expansion of the material by constructing it with an atom that has an ionic radius that relates to the co-efficient of thermal expansion you desire,” explained Peterson.
The results of this work were published in Angewandte Chemie in 2013.
“We then looked at the possibility of locking in some of these interesting and unusual coordination geometries using chemical substitutions, and we found that by incorporating potassium into the structure, this could indeed be achieved.”
“The next logical thing to do was to see if we could induce this same coordination geometry change using pressure,” said Peterson.
Applying slight pressure induces extreme compressibility
When they squashed it, the material underwent the coordination geometry change they were looking for.
They compared the compressibility of the material with a range of others, and found that it was extremely compressible —approaching the compressibility of solid polystyrene.
They both measured and then calculated the volume change using density functional theory (DFT), for a range of chemically-substituted materials, deriving the compressibility from the change in volume as a function of pressure.
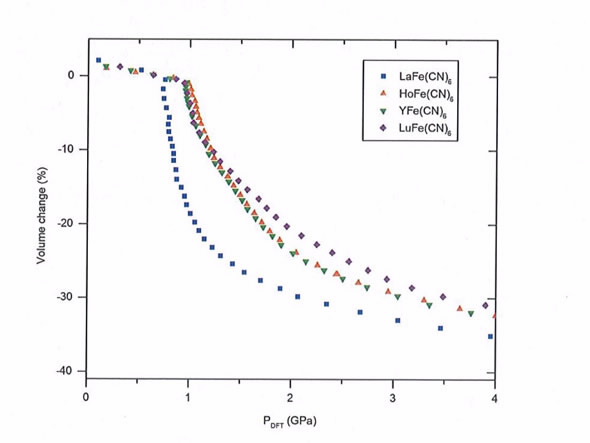 |
| Pressure-volume behaviour of the LnFe(CN)6 frameworks (Ln = La, Ho, Lu Y) as calculated using density functional theory (above) using results from neutron powder diffraction on the Wombat instrument |
“The compressibility changes as different metals are substituted into the unstable coordination geometry. This points towards tunable compressibility which is very desirable.”
They calculated the framework structure at different pressures to show exactly how the distortion happens and then calculated the contribution of each particular part of the framework in terms of energy—the energy cost of that deformation¬ and the overall contribution to the compressibility.
“Basically it’s a low energy distortion that relieves the framework strain. We showed that it can be accessed by thermal energy initially in the temperature study.”
A picture emerges of a torsion spring-like mechanism. Six-coordinate lanthanoid units act like a spring—they flex and distort as the Fe(CN)6 units act as gears.
“The energy cost of deforming the Fe(CN)6 unit in the framework is very high, so instead it is the lanthanoid unit that deforms,” said Peterson.
In addition, the lanthanoid framework has sustained axial compression, which is the largest for any crystalline solid in particularly useful regions of .4 to 1.5 GPa.
“It occurs via an interesting mechanism,” said Peterson
“There is a positive linear compressibility that goes through a small region of negative linear compressibility, where as it is squashed it gets smaller really rapidly but then bigger in that direction because of a cam-like action.”
“We examined quantitatively the energy cost of all of these components to learn exactly what they contribute to the overall volume response of the material to pressure. Once we understand the contribution that each part of the framework has to the overall properties, it can then be tuned.”
Generally speaking things will try to relieve strain. “Under pressure, the softest part with the lowest energy cost to relieve that pressure will respond.”
The team suspects that the luminescent properties of these materials are also likely to change upon compression, because the bonding changes so dramatically.
“It’s very satisfying to find a material with such interesting, useful properties, and then to establish exactly how it is they are achieved,” said Peterson.
http://dx.doi.org/10.1038/nchem.2431


