Nuclear research has provided new information about the longevity of kidney stones, one of the most common disorders of the urinary tract, affecting 1 in 10 Australian men and 1 in 35 Australian women during their lifetime.
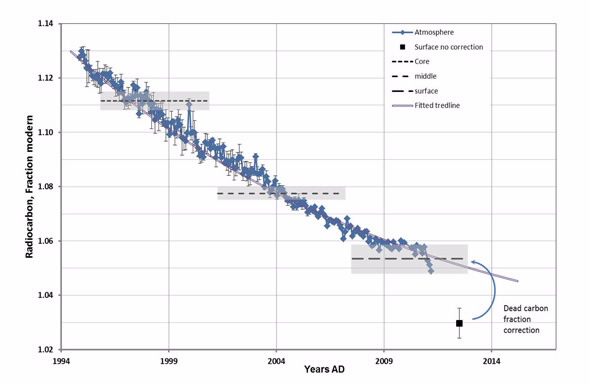 |
| Radiocarbon content of outer, middle and inner section of 17.6 year-old kidney stone plotted with Southern hemisphere atmospheric 14CO2 curve. |
“Although there is information about possible causes of kidney stones, there was relatively little known about their growth cycle,” explained Senior Research Scientist Vladimir Levchenko, who carried out the dating.
“I found that almost no one in Australia was undertaking research in this area, apart from a biomedical scientist from Adelaide, who retired shortly after I started making inquiries,” said Levchenko.
An expert in the use of accelerator mass spectrometry (AMS) using carbon for dating purposes, Levchenko considered the possibility of applying nuclear techniques to resolve the question of age.
Radiocarbon dating had been used as a biological tracer for short term biological processes in the 1970s and had been applied to measurements of human tissue after 2005.
The dating, which was undertaken at the Centre for Accelerator Science, proved to be very useful in determining the growth history of a human kidney stone and the research has been accepted by the journal Radiocarbon.
It is believed to be the first determination of the longevity of kidney stone growth and has confirmed the validity of the experimental approach.
| X-ray CT scan video of half of a 23 year old kidney stone in 3D. Red colour depicts ring of organic material. Video courtesy of Erasmus Medical Centre (Netherlands) |
Time history of a human kidney stone
Stones are classified by the type of crystal they contain. The most common type of kidney stone is made from calcium oxalate, which is formed with the combination of calcium and oxalic acid.
The oxalic acid has two carbon and four oxygen atoms. Because the sample contains carbon, the amount of its rare carbon-14 isotope can be measured using AMS.
Levchenko was able to acquire a calcium oxalate kidney stone. He set out to answer some questions about the progression of growth, differences in growth that might be due to the type of kidney stone, and if it was possible to find connections to other factors, such as nutrition or disease.
Levchenko produced three samples from the stone for the measurements: one from the surface, one of the inner intermediate section and one from the core. The stone was about 6mm in length and had a slightly irregular shape.
 |
| View of kidney stone with red dots indicating location of radial lines for sampling |
The first step was to sample the calcium oxalate by progressively dissolving it to extract the carbon. Later the oxalate was combusted to CO2 and this gas converted to graphite for isotopic carbon determinations on the accelerator. ANSTO chemist Alan Williams assisted with the chemistry for the AMS.
Measurements on the 2MV Star instrument in 2013 indicated the stone had been growing steadily for 17.6 years since 1994.
Radiocarbon dating for modern samples utilises the effect of nuclear testing in the 1950s, known as Bomb-pulse, which added sharp pulse of radioactive carbon-14 to the atmosphere.
The first result suggested that the stone had less carbon-14 than the levels in the contemporary ambient atmosphere.
Levchenko knew that this had to be explained and after exploring several ideas, he suspected that it might be due to consuming vegetables grown in greenhouses.
Vegetables take carbon atoms from the surrounding environment, which can be the greenhouse air, artificially enriched with radiocarbon-depleted CO2¬, as well as from fertiliser also containing little or no radiocarbon.1
“The finding that some tissues in our bodies may have a 2.3 per cent less carbon-14 than contemporary atmospheric levels was somewhat unexpected,” said Levchenko.
 |
|
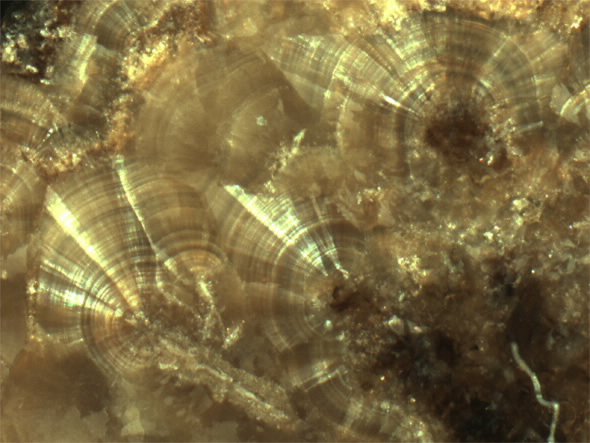
Microphotograph of interior structure of 23 year old kidney stone made of calcium oxalate. Structured pattern with growth layers visible. (50X magnification)
|
Following his initial inquiries, Levchenko started collaboration with a research group in the Netherlands, led by Dr Dirk Kok, Head of the Department of Urology, Erasmus Medical Centre, Rotterdam. He wanted to send Levchenko two samples for analysis to find out if the samples were the same age and determine if they both contained phosphate.
They provided high resolution CT scans of the stones that depicted their interior which showed they were not uniform and contained voids.
 |
|
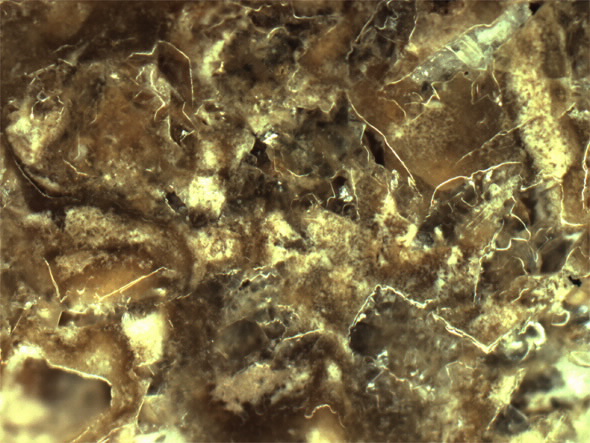
Microphotograph of interior structure of 6 year old kidney stone made of calcium phosphate. Unstructured aggregate of crystal and organic material (50X magnification)
|
Measurements on the Star accelerator indicated that one of the samples was 23 years old and the other was seven years old. The latter contained phosphorous and the other did not. Lead was found in both stones.
Linking isotope dating results with CT scans, the researchers were able to estimate the growth rate for the stones, which did vary.
As the stones were of similar size, it suggested that one was fast growing compared to the other.
As most soft drinks, especially colas, contain phosphoric acid, they were associated with the stone formation.2 Information from the two individuals who had developed the stones confirmed that one of them, the person with the fast growing stone, did regularly consume soft drinks.
Interestingly, once the age of one of the stones was determined, it was linked to a trauma that the patient had received when younger. “This connection could not have been made without dating the stone,” said Levchenko.
Dr Kok presented the research at a major urological conference in Spain in mid-September, where it was received with great interest.
Another scientist based in Australia at Macquarie University also had samples of tiny kidney stones for analysis that were mounted in resin.
Associate Professor Dorrit Jacob had undertaken previous research on the composition of fast growing kidney stones in toddlers and babies. One of the samples came from a child who had been given an inhibitor to stop stone formation.
1 J van der Plicht, JPM Beijers, Radiocarbon in food: a non-problem of health effects, Environmental Chemistry Letters, 2011 June 9 (9) 167-168
2 PM Ferrara, EN Taylor, G Gambaro, GC Curhan, Soda and other beverages and the risk of kidney stones, Clin J Am Soc Nephrology 2013 Aug 8 (8) 1389-95
Published: 01/10/2015


