Fungal spore amyloid protein, hydrophobin, is interesting because of its strong water-resistance and highly robust properties. This study investigated hydrophobin’s protein coat as there is an interest in these materials in respect to their ability to self-assemble into a robust surface layer.
Understanding the structure of this fungal spore will be useful for the design of antifungal agents such as antifungal creams or other medications. This collaborative study between ANSTO, the Universities of Sydney and New South Wales and the Harvard Medical School is the first study of its type and has pointed the way for further exploration of this material and its potential uses.
Vanessa K. Morris1, Rasmus Linser2, Karyn L. Wilde3, Anthony P. Duff3, Margaret Sunde1, Ann H. Kwan1
1 School of Medical Sciences and School of Molecular Bioscience, University of Sydney
2 School of Chemistry, University of New South Wales, and Harvard Medical School, USA,
3 ANSTO |
The water-repellent protein – hydrophin EAS
In view of supporting the design of antifungal agents the authors studied the properties of the protein coat on a fungal spore. This coat is formed by a protein, named EAS, from Neurospora crassa, which is water-repellent, i.e. hydrophobic, and classified as a class I hydrophobin.
EAS forms a layer with defined morphology that covers the spore surface, with a hydrophobic surface towards the air, and a hydrophilic surface towards the spore contents, and it serves many functional purposes. At microscopic resolution, the protein forms a structure of rodlets packed into a single-molecule thick layer as shown in Fig. 1.
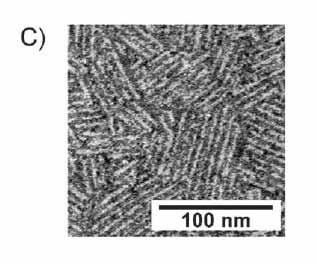 |
| Figure 1- Negatively stained transmission electronmicrograph of EASD15 rodlets. |
This layer is extremely robust, resistant to all, but the most acidic conditions, and is as hydrophobic as Teflon™ (see Fig. 2). The rodlets are insoluble and cannot be crystallised, and this severely limits the applicability of many methods to characterise them at a molecular level.
Sample preparation
The structures of the soluble form of EAS and a functional truncated variant, EASΔ15, were previously determined by solution Nuclear Magnetic Resonance (NMR) spectroscopy. The structures display a β-sheet topology characteristic to hydrophobins as can be seen in Fig. 2 [1].
 |
| Figure 2- Water droplets on bare octadecyltrichlorosilane (OTS)-treated silicon wafer and on EASΔ15-coated OTS-treated silicon wafer layer. |
The surface exhibits a clear separation of charged and hydrophobic amino acid residues that makes the proteins highly amphipathic and surface-active, illustrated in Fig.3.
The samples studied were produced by standard recombinant methods using laboratory strain E. coli. These recombinant proteins were verified as having the same functional characteristics as the native proteins, including the ability to spontaneously assemble into rodlet monolayers at hydrophobic–hydrophilic interfaces with the same regular, well-packed morphology as is observed on fungal spores and the same ability to reverse the wettability of surfaces.
Due to the insoluble and non-crystalline nature of the rodlets, solid-state NMR spectroscopy is the method of choice for studying the underlying molecular structure at atomic resolution. The method observes local atomic order within a sample without long-range order. To increase the informative signal and resolution in the NMR spectra, the samples were prepared using either double isotopic labelling with the stable isotopes carbon-13 and nitrogen-15, or triple isotopic labelling with the additional stable isotope hydrogen-2.
Hydrogen-2 (also called deuterium) labelling serves to reduce signal loss arising from the otherwise very high natural abundance of hydrogen-1. Labelling with nitrogen-15 and carbon-13 enables the sample to be probed through these atom types and increases spectral resolution.
Selective introduction of hydrogen-1 in the sample over a heavily hydrogen-2 labelled background allows detection of the sparse hydrogen-1 signals which are otherwise impossible to be observed. High yield and efficient labelling of the samples was obtained through the National Deuteration Facility at ANSTO, which has developed methods specifically for isotopic substitution in proteins.
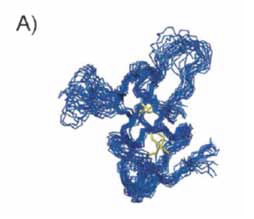 |
| Figure 3- Overlay of the 20 lowest energy structures of monomeric EASΔ15 (protein database code 2k6a). |
The measurements
A number of solid-state NMR spectra were recorded on the double- and triple-labelled samples. Specifically, the nitrogen-15/hydrogen-1 correlation spectrum recorded on EASΔ15 rodlets shows that a subset of signals stands out against a broad and poorly defined bulk.
These observations suggest that the EASΔ15 rodlets have a twofold molecular composition, with a structurally conserved and tightly packed core giving rise to the well-defined signals, among a heterogeneous ensemble of disordered regions giving rise to the broad signals.
Significantly, a comparison of this nitrogen-15/hydrogen-1 spectrum and a comparable solution spectrum recorded on EAS monomers reveals the protein fold has substantially altered upon rodlet formation. In particular, the solid-state EASΔ15 rodlet spectrum includes new signals from the amino acids glutamine or asparagine. These amino acid types are commonly found to be well-ordered in amyloid structures and have been implicated in strengthening the structure [2].
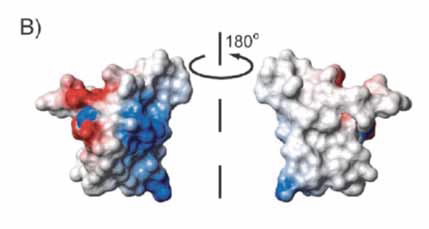 |
| Figure 4- Electrostatic surface of monomeric EASΔ15 showing positive charge in blue, and negative charge in red, and uncharged areas are white/ uncoloured. The image shows strong charge variation on one side, and nearly no charge on the other side. This causes the first side to be highly hydrophilic, and is consistent with the second side being hydrophobic. |
Identifying the structure
Despite significant disorder in the samples, signals from 18 amino acid residues could be identified in the carbon-13/carbon-13 correlation spectrum recorded on the doublelabelled rodlets. The positions of these signals were indicative of ordered β-sheet structure.
This ordered core β-sheet structure is known to be typical of amyloid structures, but the solidstate NMR data obtained here gives direct experimental evidence that this ordered β-sheet structure is new and is formed after substantial structural changes in the monomer, consistent with the hypothetical model [3,4].
In the final model, it is concluded that the rodlets are built upon a defined β-sheet core, with the remainder of the protein accommodated in many possible ways that does not affect the larger-scale rodlet-rodlet lateral packing or the overall amphipathicity of the monolayer.
The structural heterogeneity of the non-β-sheet core may offer additional functionalities and exploration of these possibilities remains part of ongoing research.
References
- A. H. Kwan, I. Macindoe, P. V. Vukasin, V. K. Morris, I. Kass, R. Gupte, A. E. Mark, M. D. Templeton, J. P. Mackay, M. Sunde, J. Mol. Biol. (2008), 382, 708 – 720.
- M. S. Dueholm, S. V. Petersen, M. Sonderkaer, P. Larsen, G. Christiansen, K. L. Hein, J. J. Enghild, J. L. Nielsen, K. L. Nielsen, P. H. Nielsen, D. E. Otzen, Mol. Microbiol. (2010), 77, 1009 – 1020.
- J. P. Mackay, J. M. Matthews, R. D. Winefield, L. G. Mackay, R. G. Haverkamp, M. D. Templeton, Structure (2001), 9, 83 – 91;
- A. H. Y. Kwan, R. D. Winefield, M. Sunde, J. M. Matthews, R. G. Haverkamp, M. D. Templeton, J. P. Mackay, Proc. Natl. Acad. Sci. USA (2006), 103, 3621 – 3626.






