A "green technology" of the leafy variety that can remove metal contamination from soils could prove to be a boon for the agricultural sector.
Microanalytical techniques provide new insight into the understanding of biological and environmental processes, such as metal hyper-accumulation in plants. Measuring the elemental distribution in different plant tissue types, such as leaves, stems and roots can help us to understand the strategies they employ to tolerate high concentrations of metals, such as Ni and Co, that are toxic to other plants.
These plants have the potential to be used to remove metal contamination from soils, a process known as phyto-remediation.
Metal accumulation in plants
Heavy metal tolerance is the ability of a plant to grow in a heavy metal-enriched soil that is toxic to most other plants.
Amazingly, some plants are tolerant species that can additionally concentrate metal(s) in their above-ground tissues to levels exceeding the concentration present in the soil or in the non-accumulating species growing nearby [1].
In some hyper-accumulating plants, the metal concentration can exceed 1000 μg g−1 (0.1%) in the dry matter of the above-ground tissue. Some plants (notably, Stackhousia tryonii) have been reported to contain exceptionally high levels of Ni (> 4%; dry weight basis).
Clearly, these properties make the plants useful in removing metal contamination from soils or to mine areas with low-yield surface deposits; technically this is called phyto-remediation of contaminated soils or phyto-mining.
Elemental imaging using nuclear methods
We can establish elemental maps of the plants using nuclear techniques such as micro Proton-Induced X-ray Emission (μ-PIXE) in order to understand the physiology of metal hyper-accumulation, and therefore study the distribution of the metals within the plant tissues.
The plants form organo-metallic complexes to hyper-accumulate toxic metals, and subsequently transport and compartmentalise these complexes within vacuoles of ‘storage’ cells. Using μ-PIXE the metals can be traced on their journey through the cell tissue.
Localisation of Ni in Hybantus Floribundus
Hybanthus floribundus, commonly known as Australian shrub violet, is one of three Ni hyper-accumulating plants reported from Australia and is one of approximately 320 Ni hyper-accumulators worldwide. It is a native Australian perennial shrub and has been reported to hyper-accumulate up to 13,500 mg Ni kg-1 of dry weight in leaf tissues.
For comparison most other plants will show signs of toxicity at Ni concentrations of 100 mg kg-1 or less.
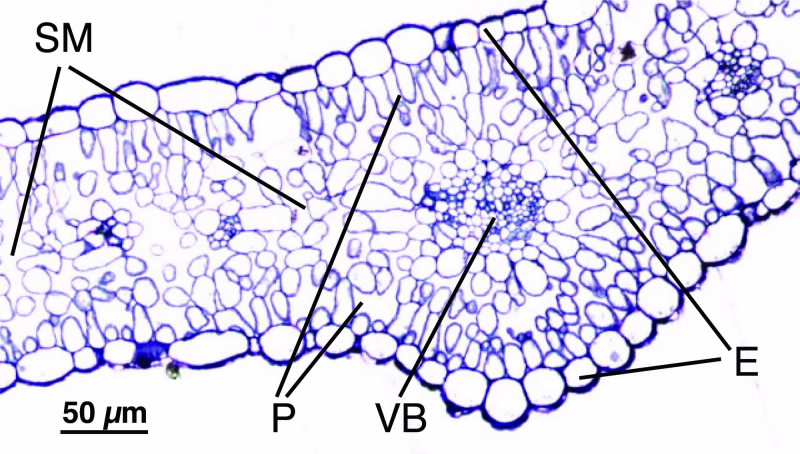 |
| Fig 1. Light micrograph of a Ni-treated leaf cross-section of Hybanthus floribundus subsp (1 um, magnification x 40); where E, epidemis, the outer layer; P, palisade mesophyll, a layer of cells just below the upper surface; SM, spongy mesophyll and VB, vascular bundle; both located in the inner part of the leaf. |
Fig. 1 shows an optical micrograph of a stained leaf section of Hybantus floribundus showing the anatomical structure of the leaf.
On both the upper and lower leaf surface a continuous single row of cells form the epidermis, outer layer. Below the epidermis is a layer of elongated cells, that form the palisade layer, which is followed by the spongy mesophyll that contains the vascular bundles, part of the plant’s transport system.
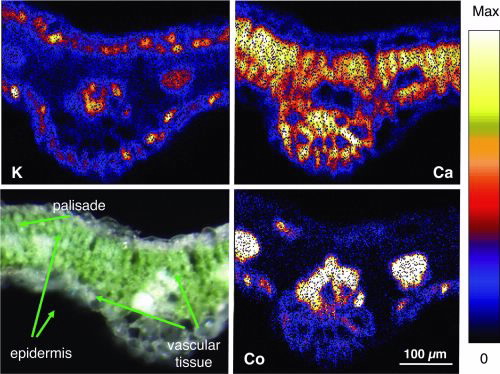
Fig. 2 shows the elemental distributions of S, Ca, K and Ni of Hybantus floribundus leaf section (Ca, K and S highlight the different structural parts of the leaf).
Lighter colours indicate a higher concentration of the element in the area. Individual cells are clearly resolved showing the variation of the concentration of elements within the cells and in different cell layers [3,4].
Ni is highly concentrated in the upper and lower epidermis of the leaf, thus accumulating in the outer layer or skin of the leaf.
However, high concentrations of Ni are also found in the vascular bundles, as Ni is being transported through the vascular tissue into the leaves. The Ni concentration in both the epidermis and the vascular tissue reaches up to 15,000 mg Ni kg-1 on a dry weight basis (yellow areas). The high-resolution images show that Ni is mainly concentrated in the walls of the epidermis cells whereas in the vascular tissue Ni is evenly distributed throughout the cells.
This information is important for plant biologists to narrow down the processes that allow the plants to transport and store the metals.
Localisation of Co in Haumaniastrum robertii
Haumaniastrum robertii is a plant that thrives in areas with high concentrations of copper and cobalt in the soil.
Its common name is copper flower, because it is often found in copper-rich soils.
However, some species can also accumulate high concentrations of cobalt, which has lead some researchers to speculate that its distribution is governed by cobalt rather then copper. Copper or cobalt concentrations in hyper-accumulating plants are much lower compared to Nickel concentrations.
 | |
|
Hence, the cobalt concentration found in this plant reaches a maximum concentration of around 2000-3000 mg kg-1. Elemental maps taken on a leaf of Haumaniastrum robertii, using the Heavy Ion Microprobe are shown in Fig. 3: High concentrations of cobalt are present in the vascular tissue, with lower concentrations in the epidermis, i.e. little accumulation of Co.
Clearly, there are different mechanisms for how these two species process these toxic metals. The cobalt results are similar to earlier findings for copper, which is also concentrated in the vascular bundles and to a lesser degree in the epidermis, indicating that in these plants the metals are to a lesser degree stored in the epidermis, but largely remain in the vascular bundles.
Further work is planned using synchrotron radiation and X-ray absorption spectroscopy (XAS) techniques which will help to better understand the molecular structure that binds the metals.
References
1. A.J.M. Baker and R.R. Brooks, Biorecovery 1 (1989) 81-126.
2. R. Siegele, N. Dytlewski and D.D. Cohen, Nucl. Instr. and Methods B158 (1999) 31-38.
3. A.G. Kachenko, B. Singh, N.P. Bhatia, R. Siegele, Nucl. Instr. and Methods B266 (2008) 667-676.
4. R. Siegele, A.G. Kachenko, M. Ionescu and D.D. Cohen, Nucl. Instr. and Methods B267 (2009) 2054-2059.


