A large international collaboration has used a specialised technique on the infrared microspectroscopy (IRM) beamline at the Australian Synchrotron to determine the structure of proteins in individual silk fibres that has potential use in the design of new biomaterials with desirable properties.
The technique, hyper-spectral infrared imaging, is a powerful analytical tool because it can establish the link between micro-/nano-structures and specific material properties of biomaterials.
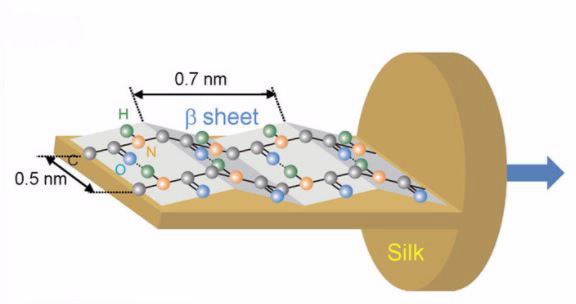 |
| The orientation of the C = O, C-N, and N-H bonds in amide structure of the L-section of silk fiber confirmed in this study by the hyper-spectral imaging |
The investigation included researchers from Swinburne University, Tokyo Institute of Technology, Deakin University, the Australian Nanofabrication Facility, rhe Centre for Physical Sciences and Technology in Lithuania, Dr Mark Tobin and Dr Pimm Vongsvivut from the Australian Synchrotron, in a study that was published in Scientific Reports.
The extraordinary properties of silk are linked to the molecular orientation of polypeptides and its amorphous/crystalline composition in the protein structure.
“The goal was to identify the orientation of proteins in different parts of the fibre and to look at how laser treatment can alter the protein structure in the silk fibre,” said Tobin, Principal Scientist ‒ IR beamline at the Australian Synchrotron.
“You would need to know the effect of a laser on silk, for example, in order to 3D print the silk,” said Tobin.
Molecular orientation is responsible for the optical, mechanical and thermal properties of biomaterials. In this study, the researchers were interested in investigating the molecular orientation of specific protein bonds in the silk that play a critical role in its strength.
Infrared imaging at the Australian Synchrotron can access molecular orientation of the protein structure directly from a single silk fibre.
“You can obtain infrared absorption information that is selected based on the orientation of a particular chemical bond,” explained Tobin.
Hyper-spectral imaging
“Because the silk fibres are only 10 microns across and the synchrotron infrared beam is about half the size of that, we developed an optical device using a germanium crystal that allowed the beam to pass through the fibre’s cross section at four times higher resolution.”
This specific device, which was developed by Vongsvivut and Tobin at the Australian Synchrotron, was recently used successfully on carbon fibres and has shown to be efficiently suitable in a broad range of applications.
Silk is a semi-crystalline material that is birefringent, which means as well as absorbing polarised light in one way it actually rotates the polarisation.
The researchers used an infrared filter to progressively rotate the polarisation of the synchrotron beam and collected four infrared (chemical) images ‒ each one with the polarisation 45 degrees apart. This unique four polarisation method was developed by the collaborative researchers in Japan. Using a mathematical formula to transform the polarisation data, they were able to work out the molecular orientation of the protein structure in the silk fibres.
Infrared imaging
In an infrared image, the intensity of the colour indicates the strength of the absorbance.
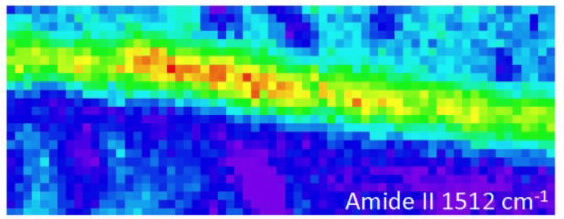 |
| High resolution 1.9 μm ATR FT-IR maps at 1.9 μm resolution of the longitudinal (L) cross sections of silk presented in auto-scale for better viewing |
“In the infrared wavelengths, you see peaks in the spectra that tell you where the light is being strongly absorbed,“ said Tobin.
“A bond vibrates at a certain energy level at a natural frequency. If light comes in at the same frequency, it can absorb some of that infrared light and vibrate to a slightly higher level,” explained Tobin.
The spectra generated in infrared images revealed that the primary vibration of the Amide II bond was all along the direction of the chain and the vibration of the Amide A bond was perpendicular to the fibre.
“With that information, our collaborators were able to work out that the protein molecules oriented in a particular way in the fibre.”
When a pulsed laser was used on one of the bonds, it disrupted the Amide A bond, changing the protein structure.
“Although the bulk information of silk fibres has probably been known, it has not been possible to measure molecular orientation on single fibres before,” said Tobin.
https://www.nature.com/articles/s41598-017-07502-3
Published: 20/09/2017


